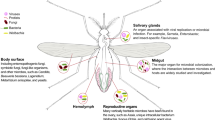
Abstract
The long-term evolutionary interaction between the host immune system and symbiotic bacteria determines their cooperative rather than antagonistic relationship. It is known that commensal bacteria have evolved a number of mechanisms to manipulate the mammalian host immune system and maintain homeostasis. However, the strategies employed by the microbiome to overcome host immune responses in invertebrates still remain to be understood. Here, we report that the gut microbiome in mosquitoes utilizes C-type lectins (mosGCTLs) to evade the bactericidal capacity of antimicrobial peptides (AMPs). Aedes aegypti mosGCTLs facilitate colonization by multiple bacterial strains. Furthermore, maintenance of the gut microbial flora relies on the expression of mosGCTLs in A. aegypti. Silencing the orthologues of mosGCTL in another major mosquito vector (Culex pipiens pallens) also impairs the survival of gut commensal bacteria. The gut microbiome stimulates the expression of mosGCTLs, which coat the bacterial surface and counteract AMP activity. Our study describes a mechanism by which the insect symbiotic microbiome offsets gut immunity to achieve homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Valiente Moro, C., Tran, F. H., Raharimalala, F. N., Ravelonandro, P. & Mavingui, P. Diversity of culturable bacteria including Pantoea in wild mosquito Aedes albopictus. BMC Microbiol. 13, 70–80 (2013).
Lemaitre, B. & Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743 (2007).
Ha, E. M., Oh, C. T., Bae, Y. S. & Lee, W. J. A direct role for dual oxidase in Drosophila gut immunity. Science 310, 847–850 (2005).
Buchon, N., Broderick, N. A. & Lemaitre, B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nature Rev. Microbiol. 11, 615–626 (2013).
Kumar, S., Molina-Cruz, A., Gupta, L., Rodrigues, J. & Barillas-Mury, C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 327, 1644–1648 (2010).
Dong, Y., Manfredini, F. & Dimopoulos, G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 5, e1000423 (2009).
Drickamer, K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J. Biol. Chem. 263, 9557–9560 (1988).
Zelensky, A. N. & Gready, J. E. The C-type lectin-like domain superfamily. FEBS J. 272, 6179–6217 (2005).
Holmskov, U., Thiel, S. & Jensenius, J. C. Collections and ficolins: humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21, 547–578 (2003).
Tailleux, L. et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197, 121–127 (2003).
Halary, F. et al. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17, 653–664 (2002).
Klimstra, W. B., Nangle, E. M., Smith, M. S., Yurochko, A. D. & Ryman, K. D. DC-SIGN, and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 77, 12022–12032 (2003).
Tassaneetrithep, B. et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197, 823–829 (2003).
Miller, J. L. et al. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 4, e17 (2008).
Waterhouse, R. M. et al. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316, 1738–1743 (2007).
Osta, M. A., Christophides, G. K. & Kafatos, F. C. Effects of mosquito genes on Plasmodium development. Science 303, 2030–2032 (2004).
Wang, Y. H. et al. A critical role for CLSP2 in the modulation of antifungal immune response in mosquitoes. PLoS Pathog. 11, e1004931 (2015).
Cheng, G. et al. A C-type lectin collaborates with a CD45 phosphatase homolog to facilitate West Nile virus infection of mosquitoes. Cell 142, 714–725 (2010).
Liu, Y. et al. Transmission-blocking antibodies against mosquito C-type lectins for dengue prevention. PLoS Pathog. 10, e1003931 (2014).
Wang, X. W., Xu, Y. H., Xu, J. D., Zhao, X. F. & Wang, J. X. Collaboration between a soluble C-type lectin and calreticulin facilitates white spot syndrome virus infection in shrimp. J. Immunol. 193, 2106–20117 (2014).
Schnitger, A. K., Yassine, H., Kafatos, F. C. & Osta, M. A. Two C-type lectins cooperate to defend Anopheles gambiae against Gram-negative bacteria. J. Biol. Chem. 284, 17616–17624 (2009).
Tanji, T., Ohashi-Kobayashi, A. & Natori, S. Participation of a galactose-specific C-type lectin in Drosophila immunity. Biochem. J. 396, 127–138 (2006).
Wang, X. W., Xu, J. D., Zhao, X. F., Vasta, G. R. & Wang, J. X. A shrimp C-type lectin inhibits proliferation of the hemolymph microbiota by maintaining the expression of antimicrobial peptides. J. Biol. Chem. 289, 11779–11790 (2014).
Suryawanshi, R. K. et al. Mosquito larvicidal and pupaecidal potential of prodigiosin from Serratia marcescens and understanding its mechanism of action. Pestic. Biochem. Physiol. 123, 49–55 (2015).
Bahia, A. C. et al. Exploring Anopheles gut bacteria for Plasmodium blocking activity. Environ. Microbiol. 16, 2980–2994 (2014).
Ramirez, J. L. et al. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS Negl. Trop. Dis. 6, e1561 (2012).
Dada, N. et al. Comparative assessment of the bacterial communities associated with Aedes aegypti larvae and water from domestic water storage containers. Parasit. Vectors 7, 391 (2014).
Gaio, A. O. et al. Use of the checkerboard DNA–DNA hybridization technique for bacteria detection in Aedes aegypti (Diptera: Culicidae) (L.). Parasit. Vectors 4, 237 (2011).
Leulier, F. et al. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nature Immunol. 4, 478–484 (2003).
Lemaitre, B., Reichhart, J. M. & Hoffmann, J. A. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl Acad. Sci. USA. 94, 14614–14619 (1997).
Geuking, M. B. et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34, 794–806 (2011).
Palm, N. W., de Zoete, M. R. & Flavell, R. A. Immune–microbiota interactions in health and disease. Clin. Immunol. 159, 122–127 (2015).
Zhou, D. et al. Cloning and characterization of prophenoloxidase A3 (proPOA3) from Culex pipiens pallens. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 162, 57–65 (2012).
Moita, L. F., Wang-Sattler, R., Michel, K., Zimmermann, T. & Blandin, S. In vivo identification of novel regulators and conserved pathways of phagocytosis in Anopheles gambiae. Immunity 23, 65–73 (2005).
Yassine, H., Kamareddine, L. & Osta, M. A. The mosquito melanization response is implicated in defense against the entomopathogenic fungus Beauveria bassiana. PLoS Pathog. 8, e1003029 (2012).
Xiao, X. P. et al. Complement-related proteins control Flavivirus infection of Aedes aegypti by inducing antimicrobial peptides. PLoS Pathog. 10, e1004027 (2014).
Liu, Y. et al. The roles of direct recognition by animal lectins in antiviral immunity and viral pathogenesis. Molecules 20, 2272–2295 (2015).
Buchon, N., Broderick, N. A., Chakrabarti, S. & Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 23, 2333–2344 (2009).
Lhocine, N. et al. PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signaling. Cell Host Microbe 4, 147–158 (2008).
Paredes, J. C., Welchman, D. P., Poidevin, M. & Lemaitre, B. Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity 35, 770–779 (2011).
Ryu, J. H. et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319, 777–782 (2008).
Guo, L., Karpac, J., Tran, S. L. & Jasper, H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell 156, 109–122 (2014).
Zaidman-Rémy, A. et al. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity 24, 463–473 (2006).
Kim, M., Lee, J. H., Lee, S. Y., Kim, E. & Chung, J. Caspar, a suppressor of antibacterial immunity in Drosophila. Proc. Natl Acad. Sci. USA. 103, 16358–16363 (2006).
Hartshorn, K. L. et al. Mechanism of binding of surfactant protein D to influenza A viruses: importance of binding to haemagglutinin to antiviral activity. Biochem. J. 351, 449–458 (2000).
Dillon, R. J. & Dillon, V. M. The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol. 49, 71–92 (2004).
Meister, S. et al. Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog. 5, e1000542 (2009).
Cirimotich, C. M. et al. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332, 855–858 (2011).
Acknowledgements
This work was funded by grants from the National Natural Science Foundation of China (81301412, 81422028, 81571975 and 61472205), the National Key Basic Research Program of the Chinese Ministry of Science and Technology (MOST) (2013CB911500), the Excellent Young Scientist Foundation of Beijing (2013D009004000002), Grand Challenges Explorations of the Bill & Melinda Gates Foundation (OPP1021992), and the National Institute of Health of the United States (AI103807 and AI099625). We thank the Professor George K. Christophides from Imperial College London, who provided critical suggestions for the manuscript. G.C. is a Newton Advanced Fellow awarded by the Academy of Medical Sciences and the Newton Fund, and a Janssen Investigator of Tsinghua University. We thank the technical supports from the Core Facility of Center for Life Sciences and Center of Biomedical Analysis (Tsinghua University).
Author information
Authors and Affiliations
Contributions
G.C. designed the experiments and wrote the manuscript; X.P. performed the majority of the experiments and analysed data; X.X., R.Z., Y. L. and J.L. helped with the RNA isolation and qPCR detection; Q.L. provided Culex pipiens pallens and contributed to the discussion. P.W. contributed experimental suggestions and strengthened the writing of the manuscript. All authors reviewed, critiqued and provided comments to the text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-8 and Tables 1-5 (PDF 1766 kb)
Rights and permissions
About this article
Cite this article
Pang, X., Xiao, X., Liu, Y. et al. Mosquito C-type lectins maintain gut microbiome homeostasis. Nat Microbiol 1, 16023 (2016). https://doi.org/10.1038/nmicrobiol.2016.23
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2016.23
This article is cited by
-
A CTL − Lys immune function maintains insect metamorphosis by preventing gut bacterial dysbiosis and limiting opportunistic infections
BMC Biology (2024)
-
34-kDa salivary protein enhances duck Tembusu virus infectivity in the salivary glands of Aedes albopictus by modulating the innate immune response
Scientific Reports (2023)
-
Microbiota in disease-transmitting vectors
Nature Reviews Microbiology (2023)
-
Antigenicity and immunogenicity of chikungunya virus-like particles from mosquito cells
Applied Microbiology and Biotechnology (2023)
-
Challenges and future directions for studying effects of host genetics on the gut microbiome
Nature Genetics (2022)