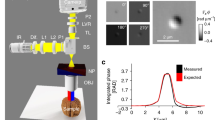
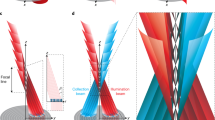
Abstract
Phase-contrast techniques, such as differential interference contrast microscopy, are widely used to obtain morphological images of unstained biological samples. The transillumination geometry required for these techniques restricts their application to thin samples. We introduce oblique back-illumination microscopy, a method of collecting en face phase-gradient images of thick scattering samples, enabling near-video-rate in vivo phase imaging with a miniaturized probe suitable for endoscopy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nomarski, G. J. Phys. Radium 16, S9–S13 (1955).
Saylor, C.F. J. Res. Natl. Bur. Stand. (US) 15, 277 (1935).
Axelrod, D. Cell Biophys. 3, 167–173 (1981).
Yi, R., Chu, K.K. & Mertz, J. Opt. Express 14, 5191–5200 (2006).
Mehta, S.B. & Sheppard, C.J.R. Opt. Lett. 34, 1924–1926 (2009).
Dubois, A. & Boccara, A.C. in Optical Coherence Tomography (eds. Drexler, W. & Fujimoto, J.G.) Ch. 19, 565–591 (Springer, 2009).
Mertz, J. Introduction to Optical Microscopy (Roberts & Company, 2009).
Groner, W. et al. Nat. Med. 5, 1209–1212 (1999).
Ford, T.N., Lim, D. & Mertz, J. J. Biomed. Opt. 17, 021105 (2012).
CUDA C Programming Guide Version 4.2. http://developer.nvidia.com/cuda/cuda-toolkit (NVIDIA, 2012).
Alerstam, E., Svensson, T. & Andersson-Engels, S. J. Biomed. Opt. 13, 060504 (2008).
Wang, L.-H., Jacques, S.L. & Zheng, L.-Q. Comput. Meth. Prog. Biomed. 47, 131–146 (1995).
Calabro, K.W., Aizenberg, E. & Bigio, I.J. Proc. SPIE 8230, 82300H (2012).
Henyey, L.G. & Greenstein, J.L. Astrophys. J. 93, 70–83 (1941).
Pogue, B.W. & Patterson, M.S. J. Biomed. Opt. 11, 041102 (2006).
Acknowledgements
We thank S. Singh (Boston University) and J. Ritt (Boston University) for supplying mouse gastrointestinal tissue samples; M. Baum (Boston University) for supplying skin and brain tissue samples; K. Calabro (Boston University) for helping develop the Monte Carlo simulation code; R. Wu (Boston University) for help with building the microscope setup used for Supplementary Figures 3 and 4; and all the members of the Biomicroscopy Lab for their helpful conversations and careful review of this manuscript. This work was supported by a US National Institutes of Health grant R01-EB010059 (T.N.F., K.K.C. and J.M.).
Author information
Authors and Affiliations
Contributions
T.N.F., K.K.C. and J.M. conceived and developed the technique. T.N.F. built the setup and acquired the data. T.N.F. and J.M. wrote the manuscript. J.M. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 519 kb)
Manual focusing through scattering tissue phantom
OBM exhibits apparent axial resolution, as demonstrated by focusing through suspended polystyrene beads. Focusing was performed by manually retracting the OBM endomicroscope probe from the sample surface. Scale bars 20 μm, imaging speed 5 Hz. (MOV 2610 kb)
CAM vasculature and demonstration of axial resolution
CAM vasculature of day 11 chick embryo visualized with OBM. Capillary vessel walls are clearly visible, as are the dynamics of individual red blood cells (RBCs). Apparent axial resolution is demonstrated by manually focusing between the CAM mesoderm and ectoderm. Our maximum imaging depth is limited here by the 60 μm working distance of our micro-objective. Scale bar 20 μm, imaging speed 17.5 Hz. (MOV 5996 kb)
CAM vasculature with moving RBCs highlighted in red
OBM video of CAM vasculature of day 11 chick embryo. Moving RBCs are highlighted in red using a sliding 3-frame temporal variance filter. Scale bar 50 μm, imaging speed 17.5 Hz. (MOV 1760 kb)
Comparison of absorption versus phase gradient images in CAM
Simultaneously acquired absorption and phase gradient OBM video of CAM vasculature of day 11 chick embryo. The probe was scanned over the sample using manually controlled translation stages. Scale bars 20 μm, imaging speed 17.5 Hz. (MOV 12419 kb)
Phase gradient mosaic of CAM vasculature
Mosaic created from OBM video of CAM vasculature of day 11 chick embryo. Scale bar 50 μm, imaging speed 17.5 Hz. (MOV 1151 kb)
Morphological features of mouse distal colon
OBM video of crypts of Lieberkühn in excised mouse distal colon. Scale bar 30 μm, imaging speed 5 Hz. (MOV 463 kb)
Morphological features of mouse small intestine
OBM video of ileal villi in excised mouse small intestine. Scale bar 30 μm, imaging speed 5 Hz. (MOV 1079 kb)
OBM reveals pyramidal neurons in thick mouse brain slice
OBM video of the CA1 region of mouse hippocampus. Pyramidal neuron somata and thick dendrites are observed in the stratum pyramidale, bordered by the stratum oriens (above) and stratum radiatum (below). Slice thickness 4.3 mm, scale bar 20 μm, imaging speed 17.5 Hz. (MOV 3870 kb)
Rights and permissions
About this article
Cite this article
Ford, T., Chu, K. & Mertz, J. Phase-gradient microscopy in thick tissue with oblique back-illumination. Nat Methods 9, 1195–1197 (2012). https://doi.org/10.1038/nmeth.2219
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2219
This article is cited by
-
Miniaturized microscope for non-invasive imaging of leukocyte-endothelial interaction in human microcirculation
Scientific Reports (2023)
-
Real-time holographic lensless micro-endoscopy through flexible fibers via fiber bundle distal holography
Nature Communications (2022)
-
Spheroscope: A custom-made miniaturized microscope for tracking tumour spheroids in microfluidic devices
Scientific Reports (2020)
-
Bond-selective transient phase imaging via sensing of the infrared photothermal effect
Light: Science & Applications (2019)
-
Epi-illumination gradient light interference microscopy for imaging opaque structures
Nature Communications (2019)