Abstract
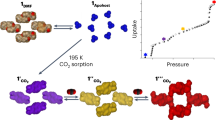
Organic solids composed by weak van der Waals forces are attracting considerable attention owing to their potential applications in gas storage, separation and sensor applications1. Herein we report a gas-induced transformation that remarkably converts the high-density guest-free form of a well-known organic host (p-tert-butylcalix[4]arene) to a low-density form and vice versa1, a process that would be expected to involve surmounting a considerable energy barrier2. This transformation occurs despite the fact that the high-density form is devoid of channels or pores3. Gas molecules seem to diffuse through the non-porous solid into small lattice voids, and initiate the transition to the low-density kinetic form with ∼10% expansion of the crystalline organic lattice, which corresponds to absorption of CO2 and N2O (refs 45). This suggests the possibility of a more general phenomenon that can be exploited to find more porous materials from non-porous organic and metal–organic frameworks that possess void space large enough to accommodate the gas molecules6,7,8,9,10,11,12,13.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Atwood, J. L. et al. Guest transport in a nonporous organic solid via dynamic van der Waals cooperativity. Science 298, 1000–1002 (2002).
Santoro, M. et al. Amorphous silica-like carbon dioxide. Nature 441, 857–860 (2006).
Dalgarno, S. J. et al. Engineering void space in organic van der Waals crystals: Calixarenes lead the way. Chem. Soc. Rev. 36, 236–245 (2007).
Mellot-Draznieks, C. et al. Very large swelling in hybrid frameworks: A combined computational and powder diffraction study. J. Am. Chem. Soc. 127, 16273–16278 (2007).
Serre, et al. Role of solvent–host interactions that lead to very large swelling of hybrid frameworks. Science 315, 1828–1830 (2007).
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Zhao, X. et al. Hysteretic adsorption and desorption of hydrogen by nanoporous metal–organic frameworks. Science 306, 1012–1015 (2004).
Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Edn 43, 2334–2375 (2004).
Zhang, J. P. et al. Temperature or guest induced drastic single-crystal-to-single-crystal transformations of a nanoporous coordination polymer. J. Am. Chem. Soc. 127, 14162–14163 (2005).
Ripmeester, J. A. et al. What we have learned from the study of solid p-tert-butylcalix[4]arene. Chem. Commun. 4986–4996 (2006).
Brouwer, E. B. et al. The complex relationship between guest-free polymorphic products and desolvation of p-tert-butylcalix[4]arene inclusion compounds. Chem. Commun. 1416–1417 (2003).
Udachin, K. A. et al. Pseudopolymorphism in the p-tert-butylcalix[4]arene–n-butylamine system: Directing the structural motif. Chem. Commun. 2162–2163 (2002).
Thallapally, P. K. et al. Acetylene absorption and binding in a nonporous crystal lattice. Angew. Chem. Int. Edn 45, 6506–6510 (2006).
Thallapally, P. K. et al. Organic crystals absorb hydrogen gas under mild conditions. Chem. Commun. 5272–5273 (2005).
Thallapally, P. K. et al. Crystal engineering of nonporous organic solids for methane sorption. Chem. Commun. 4420–4421 (2005).
Thallapally, P. K., McGrail, P. B. & Atwood, J. L. Sorption of nitrogen oxides in a nonporous crystal. Chem. Commun. 1521–1523 (2007).
Enright, G. D. et al. Thermally programmable gas storage and release in single crystals of an organic van der Waals host. J. Am. Chem. Soc. 125, 9896–9897 (2003).
Brouwer, E. B. et al. Solid-state NMR and diffraction studies of p-tert-butylcalix[4]arene.nitrobenzene.xenon. Chem. Commun. 939–940 (1997).
Kondo, A. et al. Novel expansion/shrinkage modulation of 2D layered MOF triggered by clathrate formation with CO2 molecules. Nano Lett. 6, 2581–2583 (2006).
Bradshaw, D., Warren, J. E. & Rosseinsky, M. J. Reversible concerted ligand substitution at alternating metal sites in an extended solid. Science 315, 977–980 (2007).
Polson, J. M., Fyfe, J. D. D. & Jeffrey, K. R. The reorientation of t-butyl groups in butylated hydroxytoluene: A deuterium nuclear magnetic resonance spectral and relaxation time study. J. Chem. Phys. 94, 3381–3384 (1991).
Christian, J. W. The Theory of Transformations in Metals and Alloys (Pergamon, Oxford, 1981).
Kumagai, H. et al. Facile synthesis of p-tert-butylthiacalix[4]arene by the reaction of p-tert-butylphenol with elementary sulfur in the presence of a base. Tet. Lett. 38, 3971–3972 (1997).
Department of Energy. A Research Needs Assessment for the Capture, Utilization and Disposal of Carbon Dioxide From Fossil Fuel-Fired Power Plants 61–80, DOE/ER-30194 (Massachusetts Institute of Technology, Cambridge, 1993).
Acknowledgements
This work was supported in part by Laboratory Directed Research and Development funding. In addition, portions of the work were supported by the US Department of Energy, Office of Energy Efficiency and Office of Fossil Energy. The Pacific Northwest National Laboratory is operated by Battelle for the US Department of Energy under Contract DE-AC05-76RL01830. We thank P. F. Martin and S. D. Barton for gas sorption and solid-state nuclear magnetic resonance measurements. We also thank of one of the referees for thoughts about the mechanism.
Author information
Authors and Affiliations
Contributions
P.K.T. and J.L.A. analysed and interpreted the data from H.T.S. and J.T. H.T.S. and J.T. carried out powder diffraction and single-crystal diffraction experiments. P.K.T., B.P.M., S.J.D. and J.L.A. wrote the paper.
Corresponding authors
Supplementary information
Supplementary Information
Supplementary figures S1-S22 (PDF 2309 kb)
Rights and permissions
About this article
Cite this article
Thallapally, P., Peter McGrail, B., Dalgarno, S. et al. Gas-induced transformation and expansion of a non-porous organic solid. Nature Mater 7, 146–150 (2008). https://doi.org/10.1038/nmat2097
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2097
This article is cited by
-
Nonporous amorphous superadsorbents for highly effective and selective adsorption of iodine in water
Nature Communications (2023)
-
Reversible transformations between the non-porous phases of a flexible coordination network enabled by transient porosity
Nature Chemistry (2023)
-
Applications of macrocycle-based solid-state host–guest chemistry
Nature Reviews Chemistry (2023)
-
Inclusion of organic molecule guests by sulfinyl bridged bis-salicylic acid-type open-chain host with flexible change of crystal structure
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2022)
-
Recovery of host crystals from inclusion crystals of p-tert-butylcalix[4]arene and p-tert-butylthiacalix[4]arene by the treatment with a solvent and/or supercritical CO2
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2018)