Abstract
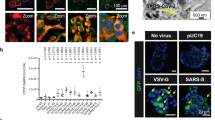
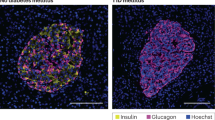
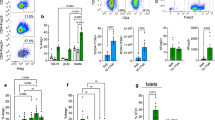
Pancreatic islets of Langerhans are enveloped by peri-islet Schwann cells (pSC), which express glial fibrillary acidic protein (GFAP) and S100β. pSC-autoreactive T- and B-cell responses arise in 3- to 4-week-old diabetes-prone non-obese diabetic (NOD) mice, followed by progressive pSC destruction before detectable β-cell death. Humans with probable prediabetes generate similar autoreactivities, and autoantibodies in islet-cell autoantibody (lCA) –positive sera co-localize to pSC. Moreover, GFAP-specific NOD T-cell lines transferred pathogenic peri-insulitis to NOD/severe combined immunodeficient (NOD/SCID) mice, and immunotherapy with GFAP or S100β prevented diabetes. pSC survived in rat insulin promoter Iymphocytic choriomeningitis virus (rip–LCMV) glycoprotein/CD8+ T-cell receptorgp double-transgenic mice with virus-induced diabetes, suggesting that pSC death is not an obligate consequence of local inflammation and β-cell destruction. However, pSC were deleted in spontaneously diabetic NOD mice carrying the CD8+/8.3 T-cell receptor transgene, a T cell receptor commonly expressed in earliest islet infiltrates. Autoimmune targeting of pancreatic nervous system tissue elements seems to be an integral, early part of natural type 1 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Serreze, D.V. & Leiter, E.H. Genetic and pathogenic basis of autoimmune diabetes in NOD mice. Curr. Opin. Immunol. 6, 900–906 (1994).
Wicker, L.S., Todd, J.A. & Peterson, L.B. Genetic control of autoimmune diabetes in the NOD mouse. Annu. Rev. Immunol. 13, 179–200 (1995).
Karges, W., Ilonen, J., Robinson, B.H. & Dosch, H.-M. Self and non-self antigen in diabetic autoimmunity: Molecules and mechanisms. Mol. Aspects Med. 16, 79–213 (1995).
Shimada, A., Charlton, B., Taylor, E.C. & Fathman, C.G. β-cell destruction may be a late consequence of the autoimmune process in nonobese diabetic mice. Diabetes 45, 1063–1067 (1996).
Atkinson, M.A. & Leiter, E.H. The NOD mouse model of type 1 diabetes: As good as it gets? Nature Med. 5, 601–604 (1999).
Tisch, R., Wang, B. & Serreze, D.V. Induction of glutamic acid decarboxylase 65-specific Th2 cells and suppression of autoimmune diabetes at late stages of disease is epitope dependent. J. Immunol. 163, 1178–1187 (1999).
Winer, S. et al. Peptide dose, MHC-affinity and target self-antigen expression are critical for effective immunotherapy of NOD mouse prediabetes. J. Immunol. 165, 4086–4094 (2000).
Andre, I. et al. Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc. Natl. Acad. Sci. USA 93, 2260–2263 (1996).
Kim, J. et al. Differential expression of GAD65 and GAD67 in human, rat, and mouse pancreatic islets. Diabetes 42, 1799–1808 (1993).
Lu, J. et al. Identification of a second transmembrane protein tyrosine phosphatase, IA-2β, as an autoantigen in insulin-dependent diabetes mellitus: Precursor of the 37-kDa tryptic fragment. Proc. Natl. Acad. Sci. USA 93, 2307–2311 (1996).
Karges, W., Pietropaolo, M., Ackerley, C. & Dosch, H.M. Gene expression of islet cell antigen p69 (ICAp69) in man, mouse and rat. Diabetes 45, 513–521 (1996).
Marrosu, M.G. et al. Patients with multiple sclerosis and risk of type 1 diabetes mellitus in Sardinia, Italy: A cohort study. Lancet 359, 1461–1465 (2002).
Winer, S. et al. Type I diabetes and MS patients target islet plus CNS-autoantigens, non-immunized NOD mice can develop autoimmune encephalitis. J. Immunol. 166, 2831–2841 (2001).
Salomon, B. et al. Development of spontaneous autoimmune peripheral polyneuropathy in b7-2-deficient nod mice. J. Exp. Med. 194, 677–684 (2001).
Donev, S.R. Ultrastructural evidence for the presence of a glial sheath investing the islets of Langerhans in the pancreas of mammals. Cell Tissue Res. 237, 343–348 (1984).
Smith, P.H. Structural modification of Schwann cells in the pancreatic islets of the dog. Am. J. Anat. 144, 513–517 (1975).
Sunami, E. et al. Morphological characteristics of Schwann cells in the islets of Langerhans of the murine pancreas. Arch. Histol. Cytol. 64, 191–201 (2001).
Teitelman, G., Guz, Y., Ivkovic, S. & Ehrlich, M. Islet injury induces neurotrophin expression in pancreatic cells and reactive gliosis of peri-islet Schwann cells. J. Neurobiol. 34, 304–318 (1998).
Winer, S. et al. ICA69null NOD mice develop diabetes but resist disease acceleration by cyclophosphamide. J. Immunol. 168, 475–482 (2002).
Saravia-Fernandez, F. et al. Localization of γ-aminobutyric acid and glutamic acid decarboxylase in the pancreas of the nonobese diabetic mouse. Endocrinology 137, 3497–3506 (1996).
Tisch, R. et al. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature 366, 72–75 (1993).
Kaufman, D.L. et al. Spontaneous loss of T cell tolerance to glutamic acid decarboxylase in murine insulin dependent diabetes. Nature 366, 69–72 (1993).
Merchant, M. & Weinberger, S.R. Recent advancements in surface-enhanced laser desorption/ionization-time of flight-mass spectrometry. Electrophoresis 21, 1164–1177 (2000).
Lipton, R.B. et al. Autoimmunity and genetics contribute to the risk of insulin-dependent diabetes mellitus in families: Islet cell antibodies and HLA DQ heterodimers. Am. J. Epidemiol. 136, 503–512 (1992).
Dosch, H.-M., Cheung, R.K., Karges, W., Pietropaolo, M. & Becker, D.J. Persistent T cell anergy in human type 1 diabetes. J. Immunol. 163, 6933–6940 (1999).
Wicker, L.S., Miller, B.J. & Mullen, Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes 35, 855–860 (1986).
DiLorenzo, T.P. et al. Major histocompatibility complex class I-restricted T cells are required for all but the end stages of diabetes development in nonobese diabetic mice and use a prevalent T cell receptor α chain gene rearrangement. Proc. Natl. Acad. Sci. USA 95, 12538–12543 (1998).
Amrani, A. et al. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 406, 739–742 (2000).
Serreze, D.V. et al. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J. Immunol. 166, 1352–1359 (2001).
Ohashi, P.S. et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65, 305–317 (1991).
Pircher, H., Burki, K., Lang, R., Hengartner, H. & Zinkernagel, R.M. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature 342, 559–561 (1989).
Verdaguer, J. et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J. Exp. Med. 186, 1663–1676 (1997).
Santamaria, P. et al. β-cell-cytotoxic CD8+ T cells from nonobese diabetic mice use highly homologous T cell receptor α-chain CDR3 sequences. J. Immunol. 154, 2494–2503 (1995).
Verdaguer, J. et al. Acceleration of spontaneous diabetes in TCR-β-transgenic nonobese diabetic mice by β-cell cytotoxic CD8+ T cells expressing identical endogenous TCR-α chains. J. Immunol. 157, 4726–4735 (1996).
Spierings, E. et al. Mycobacterium leprae-specific, HLA class II-restricted killing of human Schwann cells by CD4(+) Th1 cells: a novel immunopathogenic mechanism of nerve damage in leprosy. J. Immunol. 166, 5883–5888 (2001).
Wekerle, H., Schwab, M., Linington, C. & Meyermann, R. Antigen presentation in the peripheral nervous system: Schwann cells present endogenous myelin autoantigens to lymphocytes. Eur. J. Immunol. 16, 1551–1557 (1986).
Wong, F.S. et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nature Med. 5, 1026–1031 (1999).
Brooks-Worrell, B., Gersuk, V.H., Greenbaum, C. & Palmer, J.P. Intermolecular antigen spreading during the preclinical period of human type I diabetes. J. Immunol. 166, 5265–5370 (2001).
Mathis, D., Vence, L. & Benoist, C. β-Cell death during progression to diabetes. Nature 414, 792–798 (2001).
Delaney, C.L., Brenner, M. & Messing, A. Conditional ablation of cerebellar astrocytes in postnatal transgenic mice. J. Neurosci. 16, 6908–6918 (1996).
Bush, T.G. et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93, 189–201 (1998).
Leitenberg, D. & Bottomly, K. Regulation of naive T cell differentiation by varying the potency of TCR signal transduction. Semin. Immunol. 11, 283–292 (1999).
Hahn von Dorsche, H., Schafer, H. & Titlbach, M. Histophysiology of the obesity-diabetes syndrome in sand rats. Adv. Anat. Embryol. Cell Biol. 130, 1–95 (1994).
Austen, B.M., Frears, E.R. & Davies, H. The use of SELDI proteinchip arrays to monitor production of Alzheimer's βamyloid in transfected cells. J. Pept. Sci. 6, 459–469 (2000).
Acknowledgements
We thank M. Trucco and R. Boti for provision of purified human islets from the Pittsburgh JDRF Diabetes Center; D. Homeard for transmission electron microscopy; D. Midha, C. McKerlie and D. Winer for helpful discussions; and A. Darnley and K. Reilly for collection of human blood samples and ICA assays. This work was supported by grants from the Canadian Institutes of Health Research, the Juvenile Diabetes Research Foundation, the National Institutes of Health and the Renziehausen Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.J. and X.L., of SynX Pharma Inc., Toronto, Ontario, employed proteomics technology to make the original observation of GFAP autoantibodies in sera from humans and NOD mice with probable prediabetes. The company filed for patent protection and provided some of the funding from the present study in the form of a peer-reviewed, joint University-Industry grant from the Canadian Institutes of Health Research (CHIR). X.L. has since moved to become a Professor of Biochemistry and principal of the Biotech Center for Applied Research and Training at Seneca College, Toronto.
Rights and permissions
About this article
Cite this article
Winer, S., Tsui, H., Lau, A. et al. Autoimmune islet destruction in spontaneous type 1 diabetes is not β-cell exclusive. Nat Med 9, 198–205 (2003). https://doi.org/10.1038/nm818
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm818
This article is cited by
-
The Role of Accessory Cells in Islet Homeostasis
Current Diabetes Reports (2018)
-
Apportioning Blame: Autoreactive CD4+ and CD8+ T Cells in Type 1 Diabetes
Archivum Immunologiae et Therapiae Experimentalis (2017)
-
TRPV1 gene polymorphisms in patients with diabetes compared with healthy individuals
Comparative Clinical Pathology (2017)
-
Receptor for Advanced Glycation End Products (RAGE) in Type 1 Diabetes Pathogenesis
Current Diabetes Reports (2016)
-
Immunohistochemical Analysis and Electron Microscopy of Glial Cells in the Pancreas of Fetuses and Children
Bulletin of Experimental Biology and Medicine (2015)