Abstract
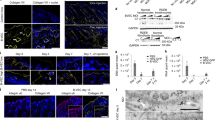
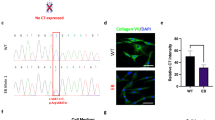
Current gene-transfer technologies display limitations in achieving effective gene delivery. Among these limitations are difficulties in stably integrating large corrective sequences into the genomes of long-lived progenitor-cell populations. Current larger-capacity viral vectors suffer from biosafety concerns, whereas plasmid-based approaches have poor efficiency of stable gene transfer1,2. These barriers hinder genetic correction of many severe inherited human diseases, such as the blistering skin disorder recessive dystrophic epidermolysis bullosa (RDEB)3, caused by mutations in the large COL7A1 gene. To circumvent these barriers, we used the φC31 bacteriophage integrase4,5,6, which stably integrates large DNA sequences containing a specific 285-base-pair attB sequence into genomic 'pseudo-attP sites'. φC31 integrase–based gene transfer stably integrated the COL7A1 cDNA into genomes of primary epidermal progenitor cells from four unrelated RDEB patients. Skin regenerated using these cells displayed stable correction of hallmark RDEB disease features, including Type VII collagen protein expression, anchoring fibril formation and dermal-epidermal cohesion. These findings establish a practical approach to nonviral genetic correction of severe human genetic disorders requiring stable genomic integration of large DNA sequences.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
23 September 2002
This was incorrect in AOP version but corrected in print. Added corrected info
References
Hengge, U.R., Walker, P.S. & Vogel, J.C. Expression of naked DNA in human, pig, and mouse skin. J. Clin. Invest. 97, 2911–2916 (1996).
Choate, K.A. & Khavari, P.A. Direct cutaneous gene delivery in a human genetic skin disease. Hum. Gene Ther. 8, 1671–1677 (1997).
Marinkovich, M.P., Herron, G.S., Khavari, P.A. & Bauer, E.A. Hereditary epidermolysis bullosa. in Dermatology in General Medicine, Vol. I (eds. Freedberg, I.M. et al.) 690–702 (McGraw-Hill, New York, 1999).
Kuhstoss, S. & Rao, R.N. Analysis of the integration function of the streptomycete bacteriophage φ C31. J. Mol. Biol. 222, 897–908 (1991).
Thyagarajan, B., Olivares, E.C., Hollis, R.P., Ginsburg, D.S. & Calos, M.P. Site-specific genomic integration in mammalian cells mediated by phage φ C31 integrase. Mol. Cell Biol. 21, 3926–3934 (2001).
Groth, A.C., Olivares, E.C., Thyagarajan, B. & Calos, M.P. A phage integrase directs efficient site-specific integration in human cells. Proc. Natl. Acad. Sci. USA 97, 5995–6000 (2000).
Sclimenti, C.R., Thyagarajan, B. & Calos, M.P. Directed evolution of a recombinase for improved genomic integration at a native human sequence. Nucleic Acids Res. 29, 5044–5051 (2001).
Christiano, A.M. et al. Structural organization of the human type VII collagen gene (COL7A1), composed of more exons than any previously characterized gene. Genomics 21, 169–179 (1994).
Barrandon, Y. & Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA 84, 2302–2306 (1987).
Medalie, D.A. et al. Evaluation of human skin reconstituted from composite grafts of cultured keratinocytes and human acellular dermis transplanted to athymic mice. J. Invest. Dermatol. 107, 121–127 (1996).
Choate, K.A., Medalie, D.A., Morgan, J.R. & Khavari, P.A. Corrective gene transfer in the human skin disorder lamellar ichthyosis. Nature Med. 2, 1263–1267 (1996).
Freiberg, R.A. et al. A model of corrective gene transfer in X-linked ichthyosis. Hum. Molec. Genet. 6, 937–933 (1997).
Seitz, C.S., Giudice, G.J., Balding, S.D., Marinkovich, M.P. & Khavari, P.A. BP180 gene delivery in junctional epidermolysis bullosa. Gene Ther. 6, 42–47 (1999).
Robbins, P.B. et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc. Natl. Acad. Sci. USA 98, 5193–5198 (2001).
Bruckner-Tuderman, L., Hopfner, B. & Hammami-Hauasli, N. Biology of anchoring fibrils: Lessons from dystrophic epidermolysis bullosa. Matrix Biol. 18, 43–54 (1999).
Heinonen, S. et al. Targeted inactivation of the type VII collagen gene (Col7a1) in mice results in severe blistering phenotype: A model for recessive dystrophic epidermolysis bullosa. J. Cell Sci. 112, 3641–3648 (1999).
Burton, E.A. & Davies, K.E. Muscular dystrophy—reason for optimism? Cell 108, 5–8 (2002).
Evans, J.T. & Garcia, J.V. Lentivirus vector mobilization and spread by human immunodeficiency virus. Hum. Gene Ther. 11, 2331–2339 (2000).
Kochanek, S. High-capacity adenoviral vectors for gene transfer and somatic gene therapy. Hum. Gene Ther. 10, 2451–2459 (1999).
Kolodka, T.M., Garlick, J.A. & Taichman, L.B. Evidence for keratinocyte stem cells in vitro: Long term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc. Natl. Acad. Sci. USA 95, 4356–4561 (1998).
Stockschlader, M.A., Schuening, F.G., Graham, T.C. & Storb, R. Transplantation of retrovirus-transduced canine keratinocytes expressing the β-galactosidase gene. Gene Ther. 1, 317–322 (1994).
Pfutzner, W., Joari, M.A., Hengge, U.R. & Vogel, J.C. A raft culture model demonstrates the feasibility of topical selection for keratinocytes expressing a desired gene. J. Invest. Dermatol. 112, 552 (1999).
Freiberg, R.A., Ho, S.N. & Khavari, P.A. Transcriptional control in keratinocytes and fibroblasts using synthetic ligands. J. Clin. Invest. 99, 2610–2615 (1997).
Mathor, M.B. et al. Clonal analysis of stably transduced human epidermal stem cells in culture. Proc. Natl. Acad. Sci. USA 93, 10371–10376 (1996).
Khavari, P.A., Peterson, C.L., Tamkun, J.W., Mendel, D.B. & Crabtree, G.R. BRG1 contains a conserved domain of the SWI2/SNF2 family necessary for normal mitotic growth and transcription. Nature 366, 170–174 (1993).
Keene, D.R., Sakai, L.Y., Lunstrum, G.P., Morris, N.P. & Burgeson, R.E. Type VII collagen forms an extended network of anchoring fibrils. J. Cell Biol. 104, 611–621 (1987).
Acknowledgements
We thank M.P. Marinkovich for antibody reagents and helpful discussions at all stages of this work; J. Vogel for COL7A1 sequences; F. Scholl, Z. Siprashvili, A. Nguyen, N. Griffiths, P. Bernstein, P.B. Robbins and other members of the Khavari laboratory for helpful discussions; and S. Tufa for technical support. This work was supported by the USVA Office of Research and Development and by NIH AR44012 (to P.A.K.) and support from the Nu Skin Center for Dermatologic Research and the Epidermolysis Bullosa Medical Research Foundation. NOTE: In the version of the article initially published online, the Acknowledgments section inadvertently omitted funding support to one of the authors. This has been corrected in the HTML and PDF version, and will appear correctly in the forthcoming print issue.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ortiz-Urda, S., Thyagarajan, B., Keene, D. et al. Stable nonviral genetic correction of inherited human skin disease. Nat Med 8, 1166–1170 (2002). https://doi.org/10.1038/nm766
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm766
This article is cited by
-
Effect of PDGF-B Gene-Activated Acellular Matrix and Mesenchymal Stem Cell Transplantation on Full Thickness Skin Burn Wound in Rat Model
Tissue Engineering and Regenerative Medicine (2021)
-
Blastocyst Formation Rate and Transgene Expression are Associated with Gene Insertion into Safe and Non-Safe Harbors in the Cattle Genome
Scientific Reports (2017)
-
Intravenously Administered Recombinant Human Type VII Collagen Derived from Chinese Hamster Ovary Cells Reverses the Disease Phenotype in Recessive Dystrophic Epidermolysis Bullosa Mice
Journal of Investigative Dermatology (2015)
-
Preconditioning of mesenchymal stem cells for improved transplantation efficacy in recessive dystrophic epidermolysis bullosa
Stem Cell Research & Therapy (2014)
-
Aminoglycosides Restore Full-length Type VII Collagen by Overcoming Premature Termination Codons: Therapeutic Implications for Dystrophic Epidermolysis Bullosa
Molecular Therapy (2014)