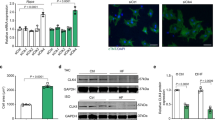
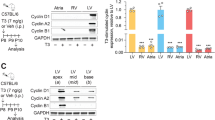
Abstract
p27Kip1 (p27) blocks cell proliferation through the inhibition of cyclin-dependent kinase-2 (Cdk2). Despite its robust expression in the heart, little is known about both the function and regulation of p27 in this and other nonproliferative tissues, in which the expression of its main target, cyclin E–Cdk2, is known to be very low. Here we show that angiotensin II, a major cardiac growth factor, induces the proteasomal degradation of p27 through protein kinase CK2-α′–dependent phosphorylation. Conversely, unphosphorylated p27 potently inhibits CK2-α′. Thus, the p27–CK2-α′ interaction is regulated by hypertrophic signaling events and represents a regulatory feedback loop in differentiated cardiomyocytes analogous to, but distinct from, the feedback loop arising from the interaction of p27 with Cdk2 that controls cell proliferation. Our data show that extracellular growth factor signaling regulates p27 stability in postmitotic cells, and that inactivation of p27 by CK2-α′ is crucial for agonist- and stress-induced cardiac hypertrophic growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
21 March 2008
In the version of this article initally published, one author, Junfeng An, was missing from the author list and the Author Contributions section. The errors have been corrected in the HTML and PDF versions of the article.
References
Berthet, C. & Kaldis, P. Cell-specific responses to loss of cyclin-dependent kinases. Oncogene 26, 4469–4477 (2007).
Koff, A., Ohtsuki, M., Polyak, K., Roberts, J.M. & Massagué, J. Negative regulation of G1 in mammalian cells: inhibition of cyclin E–dependent kinase by TGF-β. Science 260, 536–539 (1993).
Hengst, L., Dulic, V., Slingerland, J.M., Lees, E. & Reed, S.I. A cell cycle–regulated inhibitor of cyclin-dependent kinases. Proc. Natl. Acad. Sci. USA 91, 5291–5295 (1994).
Kato, J.Y., Matsuoka, M., Polyak, K., Massagué, J. & Sherr, C.J. Cyclic AMP–induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell 79, 487–496 (1994).
Polyak, K. et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78, 59–66 (1994).
Toyoshima, H. & Hunter, T. p27, a novel inhibitor of G1 cyclin–CDK protein kinase activity, is related to p21. Cell 78, 67–74 (1994).
Pagano, M. et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269, 682–685 (1995).
Hengst, L. & Reed, S.I. Translational control of p27Kip1 accumulation during the cell cycle. Science 271, 1861–1864 (1996).
Tomoda, K., Kubota, Y. & Kato, J. Degradation of the cyclin-dependent- kinase inhibitor p27Kip1 is instigated by Jab1. Nature 398, 160–165 (1999).
Hara, T. et al. Degradation of p27(Kip1) at the G0-G1 transition mediated by a Skp2-independent ubiquitination pathway. J. Biol. Chem. 276, 48937–48943 (2001).
Kamura, T. et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27Kip1 at G1 phase. Nat. Cell Biol. 6, 1229–1235 (2004).
Kotoshiba, S., Kamura, T., Hara, T., Ishida, N. & Nakayama, K.I. Molecular dissection of the interaction between p27 and KPC, the ubiquitin ligase that regulates proteolysis of p27 in G1 phase. J. Biol. Chem. 280, 17694–17700 (2005).
Ishida, N. et al. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J. Biol. Chem. 277, 14355–14358 (2002).
Kotake, Y., Nakayama, K., Ishida, N. & Nakayama, K.I. Role of serine 10 phosphorylation in p27 stabilization revealed by analysis of p27 knock-in mice harboring a serine 10 mutation. J. Biol. Chem. 280, 1095–1102 (2005).
Besson, A. et al. A pathway in quiescent cells that controls p27Kip1 stability, subcellular localization, and tumor suppression. Genes Dev. 20, 47–64 (2006).
Rodier, G. et al. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 20, 6672–6682 (2001).
LaBaer, J. et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11, 847–862 (1997).
Sheaff, R.J. et al. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11, 1464–1478 (1997).
Carrano, A.C., Eytan, E., Hershko, A. & Pagano, M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1, 193–199 (1999).
Montagnoli, A. et al. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 13, 1181–1189 (1999).
Nakayama, K. et al. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev. Cell 6, 661–672 (2004).
Aleem, E., Kiyokawa, H. & Kaldis, P. Cdc2–cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 7, 831–836 (2005).
Malek, N.P. et al. A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature 413, 323–327 (2001).
Fero, M.L. et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell 85, 733–744 (1996).
Kiyokawa, H. et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell 85, 721–732 (1996).
Nakayama, K. et al. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85, 707–720 (1996).
Loda, M. et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat. Med. 3, 231–234 (1997).
Liang, J. et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat. Med. 8, 1153–1160 (2002).
Shin, I. et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27Kip1 at threonine 157 and modulation of its cellular localization. Nat. Med. 8, 1145–1152 (2002).
Viglietto, G. et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27Kip1 by PKB/Akt-mediated phosphorylation in breast cancer. Nat. Med. 8, 1136–1144 (2002).
Pasumarthi, K.B.S. & Field, L.F. Cardiomyocyte cell cycle regulation. Circ. Res. 90, 1044–1054 (2002).
Burton, P.B., Yacoub, M.H. & Barton, P.J. Cyclin-dependent kinase inhibitor expression in human heart failure. A comparison with fetal development. Eur. Heart J. 20, 604–611 (1999).
Poolman, R.A., Li, J.M., Durand, B. & Brooks, G. Altered expression of cell cycle proteins and prolonged duration of cardiac myocyte hyperplasia in p27Kip1 knockout mice. Circ. Res. 85, 117–127 (1999).
Sadoshima, J., Xu, Y., Slayter, H.S. & Izumo, S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 75, 977–984 (1993).
Chien, K.R. & Olson, E.N. Converging pathways and principles in heart development and disease. Cell 110, 153–162 (2002).
Litchfield, D.W. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 369, 1–15 (2003).
Meggio, F. & Pinna, L.A. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 17, 349–368 (2003).
Luo, Y., Hurwitz, J. & Massagué, J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature 375, 159–161 (1995).
Grimmler, M. et al. Cdk inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128, 269–280 (2007).
Chu, I. et al. p27 phosphorylation by Src regulates inhibition of cyclin E–Cdk2. Cell 128, 281–294 (2007).
Nagahara, H. et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 4, 1449–1452 (1998).
Hunter, J.J. & Chien, K.R. Signaling pathways for cardiac hypertrophy and failure. N. Engl. J. Med. 341, 1276–1283 (1999).
Hirota, H. et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell 97, 189–198 (1999).
Satoh, M. et al. Requirement of Rac1 in the development of cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 103, 7432–7437 (2006).
Li, P.F. et al. Phosphorylation by protein kinase CK2: a signaling switch for the caspase-inhibiting protein ARC. Mol. Cell 10, 247–258 (2002).
Donath, S. et al. Apoptosis repressor with caspase recruitment domain is required for cardioprotection in response to biomechanical and ischemic stress. Circulation 113, 1203–1212 (2006).
Seldin, D.C. & Leder, P. Casein kinase IIα transgene–induced murine lymphoma: relation to theileriosis in cattle. Science 267, 894–897 (1995).
Xu, X., Toselli, P.A., Russell, L.D. & Seldin, D.C. Globozoospermia in mice lacking the casein kinase IIα′ catalytic subunit. Nat. Genet. 23, 118–121 (1999).
Kato, T. Jr, Delhase, M., Hoffmann, A. & Karin, M. CK2 is a C-terminal IκB kinase responsible for NF-κB activation during the UV response. Mol. Cell 12, 829–839 (2003).
Scaglioni, P.P. et al. A CK2-dependent mechanism for degradation of the PML tumor suppressor. Cell 126, 269–283 (2006).
Acknowledgements
We thank D.W. Litchfield (The University of Western Ontario) for the gift of antibodies to CK2-α′ and CK2-β subunits. This research was supported by Canadian Institutes of Health Research (MOP-81194) and grants from the Deutsche Forschungsgemeinschaft (Ha-1777/7-3, Ha-1777/9-1) to R.v.H., the Volkswagen-Stiftung (Lichtenberg program) and Bundesministerium für Bildung und Forschung (Center for Stroke Research Berlin) to M.E.
Author information
Authors and Affiliations
Contributions
J.R. performed the two-hybrid screen and contributed to the in vitro experiments. C.H., K.G. and M.N. performed some of the animal experiments and contributed to the in vivo analysis of the mice. J.A. contributed to the generation of recombinant TAT proteins. L.H., R.D. and R.v.H. carried out experimental design, data analysis and manuscript preparation. R.v.H. supervised L.H. and J.R.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–3, Supplementary Tables 1–3 and Supplementary Methods (PDF 578 kb)
Rights and permissions
About this article
Cite this article
Hauck, L., Harms, C., An, J. et al. Protein kinase CK2 links extracellular growth factor signaling with the control of p27Kip1 stability in the heart. Nat Med 14, 315–324 (2008). https://doi.org/10.1038/nm1729
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1729
This article is cited by
-
Protein kinase CK2: a potential therapeutic target for diverse human diseases
Signal Transduction and Targeted Therapy (2021)
-
MicroRNA-221-3p is related to survival and promotes tumour progression in pancreatic cancer: a comprehensive study on functions and clinicopathological value
Cancer Cell International (2020)
-
miR-221 and -222 target CACNA1C and KCNJ5 leading to altered cardiac ion channel expression and current density
Cellular and Molecular Life Sciences (2020)
-
Mechanisms of physiological and pathological cardiac hypertrophy
Nature Reviews Cardiology (2018)
-
Modulation of transcriptional mineralocorticoid receptor activity by casein kinase 2
Scientific Reports (2017)