Abstract
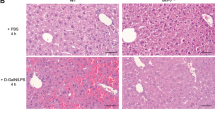
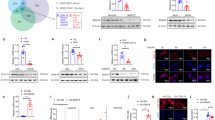
Hepatocytes in fatty livers are hypersensitive to apoptosis and undergo escalated apoptotic activity via death receptor–mediated pathways, particularly that of Fas-FasL, causing hepatic injury that can eventually proceed to cirrhosis and end-stage liver disease. Here we report that the hepatocyte growth factor receptor, Met, plays an important part in preventing Fas-mediated apoptosis of hepatocytes by sequestering Fas. We also show that Fas antagonism by Met is abrogated in human fatty liver disease (FLD). Through structure-function studies, we found that a YLGA amino-acid motif located near the extracellular N terminus of the Met α-subunit is necessary and sufficient to specifically bind the extracellular portion of Fas and to act as a potent FasL antagonist and inhibitor of Fas trimerization. Using mouse models of FLD, we show that synthetic YLGA peptide tempers hepatocyte apoptosis and liver damage and therefore has therapeutic potential.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Canbay, A., Friedman, S. & Gores, G.J. Apoptosis: the nexus of liver injury and fibrosis. Hepatology 39, 273–278 (2004).
Feldstein, A.E. et al. Hepatocyte apoptosis and Fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125, 437–443 (2003).
Feldstein, A.E. et al. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J. Hepatol. 39, 978–983 (2003).
Meagan, J. et al. Steatosis and liver cell apoptosis in chronic hepatitis C: a mechanism for increased liver injury. Hepatology 39, 1230–1238 (2004).
Browning, J.D. & Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114, 147–152 (2004).
Diehl, A.M. et al. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut 54, 303–306 (2005).
Wang, X. et al. A mechanism of cell survival: sequestration of Fas by the HGF receptor. Met. Mol. Cell 9, 411–421 (2002).
Huh, C.-G. et al. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc. Natl. Acad. Sci. USA 101, 4477–4482 (2004).
Birchmeier, C., Birchmeier, W., Gherardi, E. & Vande Woude, G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell. Biol. 4, 915–925 (2003).
Trusolino, L. & Comoglio, P.M. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat. Rev. Cancer 2, 289–300 (2002).
Gomez-Lechon, M.J. et al. A human hepatocellular in vitro model to investigate steatosis. Chem. Biol. Interact. 165, 106–616 (2007).
Nehra, V., Angulo, P., Buchman, A.L. & Lindor, K.D. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig. Dis. Sci. 46, 2347–2352 (2001).
Moriya, K. et al. Increase in the concentration of carbon 18 monounsaturated fatty acids in the liver with hepatitis C: analysis in transgenic mice and humans. Biochem. Biophys. Res. Commun. 281, 1207–1212 (2001).
Locksley, R.M., Killeen, N. & Lenardo, M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 (2001).
Bodmer, J.L., Schneider, P. & Tshopp, J. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 27, 19–26 (2002).
Starling, G.C. et al. Identification of amino acid residues important for ligand binding to Fas. J. Exp. Med. 185, 1487–1492 (1997).
Connolly, K. et al. In vivo inhibition of Fas ligand-mediated killing by TR6, a Fas ligand decoy receptor. J. Pharmacol. Exp. Ther. 298, 25–33 (2001).
Hasegawa, A. et al. Fas-disabling small exocyclic peptide mimetics limit apoptosis by an unexpected mechanism. Proc. Natl. Acad. Sci. USA 101, 6599–6604 (2004).
Yin, X.M. & Ding, W.X. Death receptor activation-induced hepatocyte apoptosis and liver injury. Curr. Mol. Med. 3, 491–508 (2003).
Li, Z. et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 37, 343–50 (2003).
Ding, X. et al. Exendin-4, a glucagon-like protein (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 43, 173–181 (2006).
Anstee, Q.M. & Goldin, R.D. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Pathol. 87, 1–16 (2006).
Kirsch, R. et al. Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J. Gastroenterol. Hepatol. 18, 1272–1382 (2003).
Leon, A.L. & Rojkind, M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J. Histochem. Cytochem. 33, 737–743 (1985).
Feldstein, A. & Gores, G.J. Steatohepatitis and apoptosis: therapeutic implications. Am. J. Gastroenterol. 99, 1718–1719 (2004).
Guicciardi, M.E. & Gores, G.J. Cheating death in the liver. Nat. Med. 10, 587–588 (2004).
Song, E. et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 9, 347–351 (2003).
Zender, L. et al. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc. Natl. Acad. Sci. USA 100, 7797–7802 (2003).
Farrell, G.C. & Larter, C.Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43, S99–S112 (2006).
Takeda, T. et al. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc. Natl. Acad. Sci. USA 97, 5498–5503 (2000).
Michalopoulos, G.K. & DeFrances, M.C. Liver regeneration. Science 276, 60–66 (1997).
Taub, R. Liver regeneration: from myth to mechanism. Nat. Rev. Mol. Cell Biol. 5, 836–847 (2004).
Ueki, T. et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat. Med. 5, 226–230 (1999).
Corso, S., Comoglio, P.M. & Giordano, S. Cancer therapy: can the challenge be MET? Trends Mol. Med. 11, 284–292 (2005).
Nakamoto, Y. et al. Prevention of hepatocellular carcinoma development associated with chronic hepatitis by anti-Fas ligand antibody therapy. J. Exp. Med. 196, 1105–1111 (2002).
Acknowledgements
We thank M. Lenardo and R. Siegel (US National Institutes of Health) for providing us with CFP and YFP-tagged Fas expression vectors, and C. Dai and X. He for their assistance. This work was supported by an R01 grant from National Institutes of Health (R01CA095782) awarded to R.Z.
Author information
Authors and Affiliations
Contributions
C.Z. performed most of the experimental work, in particular mapping of the Fas binding site on Met and its anti-apoptotic characterization using in vitro and in vivo models, and also prepared the figures and helped with manuscript writing. J.M., X.W. and Z.Z. contributed to the assessment of Met-Fas complex formation in human fatty liver. L.G. established Met knockdown by siRNA in HepG2 cells. J.S. and A.E.E. performed most aspects of the animal studies, including peptide injection and tissue collection. C.J.J. performed the in vitro studies of YLGA peptide effects on Met activation by HGF and Met signaling in Hepa1-6 cells. S.S. collected human liver specimens and prepared primary cultures of human hepatocytes. G.K.M. provided primary cultures of mouse hepatocytes and also offered invaluable discussion. M.C.D. contributed critically to the histological evaluation of liver tissues, oversaw the BIAcore experiments and assisted with results interpretation. R.Z. formulated the hypothesis that Fas antagonism by Met may be deranged in fatty liver disease, designed nearly all of the experimental procedures and finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–7 and Supplementary Methods (PDF 2004 kb)
Rights and permissions
About this article
Cite this article
Zou, C., Ma, J., Wang, X. et al. Lack of Fas antagonism by Met in human fatty liver disease. Nat Med 13, 1078–1085 (2007). https://doi.org/10.1038/nm1625
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1625
This article is cited by
-
Hepatocyte growth factor-mediated apoptosis mechanisms of cytotoxic CD8+ T cells in normal and cirrhotic livers
Cell Death Discovery (2023)
-
Understanding lipotoxicity in NAFLD pathogenesis: is CD36 a key driver?
Cell Death & Disease (2020)
-
Fas cell surface death receptor controls hepatic lipid metabolism by regulating mitochondrial function
Nature Communications (2017)
-
A multi-component classifier for nonalcoholic fatty liver disease (NAFLD) based on genomic, proteomic, and phenomic data domains
Scientific Reports (2017)
-
Experimental liver fibrosis research: update on animal models, legal issues and translational aspects
Fibrogenesis & Tissue Repair (2013)