Abstract
Identification of pathogen-specific T cells has been greatly facilitated by the advent of synthetic peptide–major histocompatibility complex (MHC) tetramers. In many cases, however, specific epitopes have not been defined, necessitating detection methods that function independently of exact peptide-MHC specificity. Lymphocytes acquire surface proteins from antigen-presenting cells (APCs), and we have exploited this phenomenon to develop the T-cell recognition of APCs by protein transfer (TRAP) assay. This method is based on biotinylation and streptavidin-fluorochrome labeling of APCs, followed by subsequent acquisition of this label by antigen-specific T cells. The TRAP procedure detects MHC class I–restricted T cells regardless of their cytokine profiles or peptide-MHC affinities, and provides a versatile tool for monitoring the phenomenon of APC membrane acquisition by antigen-specific T cells.
Similar content being viewed by others
Main
Analysis of pathogen-specific T cells requires specialized reagents to identify and isolate antigen-specific cells within a mixed lymphocyte population. Chief among these are peptide-MHC tetramers1,2. In outbred populations, however, lack of knowledge of peptide-MHC specificity precludes tetramer production and analysis. This has led to the development of cytokine-based assays. Virus-infected APCs or peptide-coated APCs are used to activate antigen-specific T cells, which are subsequently identified by their production of cytokines such as interferon (IFN)-γ using intracellular cytokine staining (ICCS), enzyme-linked immunospot (ELISPOT) or cytokine-capture assays2,3,4,5. In addition to cytokine production, transfer of cell membranes from APCs to T cells is well documented6,7,8,9,10,11,12,13,14,15,16, but little is known about the acquisition of labeled APC membranes or surface proteins by nontransgenic, polyclonal T cells analyzed directly ex vivo. Although acquisition of MHC–green fluorescent protein (GFP) fusion proteins from genetically engineered APCs is effective for quantifying pathogen-specific T cells16, this approach is limited by (i) the cell types that can be transfected, (ii) the limited color spectrum of GFP and related proteins, and (iii) the restriction to a single MHC per construct. As most inbred mice express more than one MHC, analysis based on any single haplotype measures only a selected proportion of total T-cell responses. To overcome these limitations, we developed a simple and versatile method to detect pathogen-specific T cells without a priori knowledge of peptide specificity or MHC restriction elements.
Results
TRAP: a new method of T-cell quantification
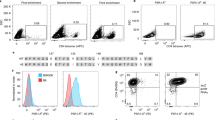
To perform the TRAP assay, we surface biotinylated APCs, labeled them with streptavidin-fluorochrome and, after 4–6 h of incubation with T cells, measured the transfer of fluorescent label to antigen-specific T cells by flow cytometry. To study and validate the TRAP method, we used a mouse model involving lymphocytic choriomeningitis virus (LCMV) infection. The immunodominant H-2Ld–restricted T-cell epitope NP118–126 (NP118) was used in these studies, and NP118-tetramer stained approximately 17% of CD8+ T cells from an LCMV-immune mouse (Fig. 1a). In vitro stimulation with NP118-coated APCs induced IFN-γ production by a comparable percentage of T cells, consistent with previous results demonstrating a correlation between NP118-tetramer staining and peptide-induced IFN-γ (ref. 2). Peptide stimulation also induced acquisition of TRAP fluorochrome label by ∼17% of CD8+ T cells, which are primarily IFN-γ+; this indicates that these assays measure the same general T-cell populations. In the experiments described here, we labeled biotinylated APCs with streptavidin–Pacific Blue, but we obtained similar results with other streptavidin reagents (streptavidin conjugated to fluorescein isothiocyanate, allophycocyanin, phycoerythrin or other fluorochromes; Supplementary Fig. 1 online). This illustrates one advantage of this approach to T-cell quantification: virtually any fluorochrome may be used to label APCs (and, subsequently, antigen-specific T cells). This adds versatility when performing multiparameter flow cytometry because it is not limited to a single color, as is the case with GFP-MHC–transfected APCs.
(a) FACS dotplots of splenocytes isolated from LCMV-immune or naive mice and stained with NP118-tetramer or activated with Pacific Blue–labeled, NP118-coated APCs. We identified activated virus-specific CD8+ T cells by IFN-γ ICCS, or by the TRAP assay to detect acquisition of APC surface label. (b) FACS dotplots of splenocytes from LCMV-immune or naive animals incubated with fluorochrome-labeled LCMV-infected or uninfected APCs. We identified virus-specific CD8+ T cells by IFN-γ production or the TRAP assay. Dotplots are pre-gated on CD8+ lymphocytes and the numbers indicate values determined after subtracting background events obtained from parallel cultures incubated with uncoated or uninfected APCs. In b, the percentages of IFN-γ+ or TRAP+ cells in unstimulated cultures are shown in parentheses. Data are representative of at least three independent experiments.
For the TRAP assay to be most versatile, it should detect pathogen-specific T cells in cases in which specific peptide epitopes have not been defined. We therefore detected virus-specific T cells via the TRAP assay using APCs directly infected with LCMV (Fig. 1b). The frequency of LCMV-specific CD8+ T cells identified using TRAP was comparable to that detected by intracellular IFN-γ production (18.6% versus 20.6%, respectively). Moreover, LCMV-infected APCs consistently induced a higher frequency of IFN-γ+ and TRAP+ T cells than NP118-peptide coated targets analyzed in parallel. NP118 peptide stimulation accounted for 82.9 ± 12.3% (mean ± s.d.) of IFN-γ+ responses elicited by LCMV-infected APCs (P = 0.0003) and 82.3 ± 10.5% of the TRAP+ responses induced by LCMV-infected APCs (P = 0.003). Virus infection allowed quantification of total virus-specific T-cell responses, including subdominant GP99–H-2Kd, GP283–H-2Kd, NP313–H-2Ld and NP314–H-2Kd peptide epitopes in addition to the immunodominant NP118–H-2Ld epitope. This shows that the TRAP assay works well with virus-infected APCs, and does not require specific peptides to be identified for direct quantification of T-cell responses to multiple peptide-MHC antigens.
Linear range of T-cell quantification by the TRAP assay
Antiviral T-cell responses to LCMV are robust and are probably due, at least in part, to the tropism of the virus: LCMV infects lymphoid tissues such as the spleen, and this is where a high proportion of memory T cells reside. Peak CD8+ T-cell responses against vaccinia also reach ∼25% of total splenic T cells3, and studies of other models have found high numbers of virus-specific T cells in nonlymphoid organs17. Likewise, if one compares the frequency of virus-specific T cells in the lungs after respiratory infection18,19,20 or in the brain after infection with neurotropic viruses21,22, it becomes clear that LCMV is not alone in inducing increases in virus-specific T cells to numbers that may reach 25–80% of the total T-cell population at sites of viral replication. Nevertheless, the question arose as to whether the TRAP assay would be sufficiently sensitive to detect less prominent memory T-cell populations. We evaluated sensitivity of CD8+ T-cell detection by mixing twofold serial dilutions of LCMV-immune splenocytes with naive splenocytes to reduce the size of the memory T-cell pool (Fig. 2). This resulted in individual samples containing a range of memory T cells that could be used to validate the quantitative range of the TRAP assay. We coated APCs with NP118 peptide (Fig. 2a) or infected them with LCMV (Fig. 2b), and we determined the frequencies of TRAP+ and IFN-γ+ T cells in each sample dilution. A plot of IFN-γ+ versus TRAP+ T cells showed a strong correlation when either peptide-coated or LCMV-infected APCs were used (R2 = 0.99; P < 0.0001). Typically, we observed that 75–90% of IFN-γ+ memory T cells were detectable by the TRAP assay (similar to NP118-tetramer staining; Fig. 1a), and antigen-specific cells were detected at frequencies as low as 0.5% of total CD8+ T cells (Fig. 2 insets). We obtained similar results using an alternative approach to determining the linear range of T-cell quantification in which T-cell responses to dominant and subdominant H-2b epitopes were measured in LCMV-immune C57BL/6 mice (Supplementary Fig. 2 online). In this case, the frequency of memory T cells specific for LCMV peptides GP33, NP396 and GP276 ranged from ∼1% to 7%, with a clear linear correlation between the number of T cells measured by IFN-γ ICCS and that measured by the TRAP assay (R2 = 0.94; P = 0.0003).
(a,b) FACS analysis of splenocytes isolated from four LCMV-immune mice at the indicated times after infection that were used directly or serially diluted with naive splenocytes to decrease the frequency of memory T cells before incubation with NP118-coated APCs (a) or LCMV-infected APCs (b). Data represent combined results from two independent experiments, and numbers indicate values determined after subtracting background events obtained from parallel cultures incubated with uncoated or uninfected APCs. (c) Memory T cells are activated to produce IFN-γ and acquire surface proteins specifically from APCs infected with homologous virus. FACS dotplots of splenocytes isolated from a representative LCMV-immune (99 d after infection, left panels) or vaccinia virus (VV)-immune (33 d after infection, right panels) mouse, incubated with fluorochrome-labeled APCs infected with either LCMV or vaccinia (as indicated). Dotplots depict IFN-γ and TRAP staining of CD8+CD11ahi T cells. Numbers indicate values determined after subtracting background events obtained from parallel cultures incubated with uninfected APCs. Data are representative of two or three mice per group.
To determine whether APC membrane acquisition by T cells was indeed antigen specific and not due to an unrecognized nonspecific effect of LCMV infection, peptide exposure or damage to the APC, we measured T-cell responses after incubation with APCs infected with an irrelevant pathogen, vaccinia virus (Fig. 2c). LCMV-immune T cells became IFN-γ+ and TRAP+ in the presence of the correct cognate antigen (LCMV-infected APCs) but did not show these responses following incubation with vaccinia virus-infected APCs. Likewise, vaccinia virus–specific memory T cells responded only to vaccinia virus–infected targets by becoming IFN-γ+ and TRAP+; they were not activated by exposure to LCMV-infected APCs. This demonstrates that the TRAP assay is antigen specific and can be used to detect T-cell responses to LCMV-specific or vaccinia virus–specific T-cell antigens.
Choice of APC and membrane transfer to memory T cells
The type of APC used for the TRAP assay can play an important role in the amount of APC membrane that is transferred to antigen-specific T cells. We compared NP118-coated naive, autologous spleen cells to A20 cells (a B-cell line), J774 cells (a macrophage cell line) and BALBcl7 cells (a fibroblast cell line) (Fig. 3). Naive spleen cells represented highly effective targets for the TRAP assay, indicating that autologous primary cells work well for this application. A20 cells were also effective targets (Fig. 1, Fig. 3 and Supplementary Fig. 3 online), whereas J774 cells provided an intermediate degree of APC membrane transfer. BALBcl7 cells induce strong IFN-γ production by virus-specific T cells and have been used routinely as targets for LCMV-specific chromium-release assays23, but did not reproducibly elicit readily detectable levels of APC membrane transfer to T cells. Together, these results indicate that primary lymphocytes and B-cell or macrophage cell lines are more effective APCs in this assay than fibroblasts.
FACS histograms of fluorochrome acquisition by memory T cells from biotinylated naive splenocytes or A20, J774 or BALBcl7 cells, labeled with fluorochrome and coated with NP118 peptide (thick black line). Control fluorochrome-labeled APCs were not coated with peptide, and were incubated with responder splenocytes in the absence (solid gray line) or presence (dashed gray line) of IL-12 plus IL-18. Histograms are pre-gated on CD8+CD11ahiIFN-γ+ cells (samples stimulated with NP118 or with IL-12 plus IL-18) or CD8+CD11ahi cells (unstimulated samples). Data are representative of at least two independent experiments, with two or three mice per group.
Discussion
The TRAP assay is based on APC surface-label acquisition and is comparable to peptide-tetramer staining or IFN-γ ICCS as a means of quantifying virus-specific CD8+ T cells, but with the added advantage of not requiring knowledge of strict peptide-MHC specificity. By simultaneously quantifying T-cell responses to all relevant peptide-MHC specificities, the TRAP assay may act as a universal procedure for identifying pathogen-specific T-cell populations. A previous study generated GFP-HLA-A*201–expressing APCs to measure human T-cell responses to peptide antigens that bound this single MHC molecule16; its results indicate that APC membrane acquisition by T cells is not unique to mice. It is likely that the TRAP assay will be useful for measuring broader T-cell responses to multiple peptide-MHCs in humans and other outbred populations and may have potential for measuring tumor-specific T-cell responses as well. As untransformed and untransfected autologous cells can be used as APCs, the TRAP approach may set the stage for effective sorting and in vitro culture of purified human T cells for use in immunotherapeutic applications.
Notably, T-cell activation alone is not enough to trigger uptake of APC membranes by memory T cells. Cytokine-mediated activation by IL-12 and IL-18 triggered little or no APC membrane transfer, even though these two cytokines stimulate high IFN-γ production by CD8+ T cells26,27 (Fig. 3 and Supplementary Fig. 1 online). Although the TRAP assay is antigen restricted and specific (Fig. 2), attempts to use IFN-γ–based cytokine capture assays4,24,25 can potentially be altered by the 'bystander' effect of T-cell stimulation by APC-derived cytokines such as IL-12 and IL-18 (refs. 26,27). Similarly, methods based on the activation marker CD154 will detect both antigen-stimulated28 and cytokine-stimulated27 T cells. This caveat is especially relevant if unpurified or semipurified microbial antigens are used for T-cell activation, as these may trigger innate production of cytokines including IL-12 and IL-18 (ref. 26) following Toll-like receptor signaling. This may result in lower specificity following fluorescence-activated cell sorting (FACS), although it may still be useful for enrichment of antigen-specific T cells. Another caveat is that cytokine responses of pathogen-specific T cells may be markedly altered during persistent infection29, thus further complicating T-cell quantification based on the production of a single cytokine30.
It will be interesting to determine whether T-cell acquisition of APC membrane varies during the course of acute or chronic infection. Preliminary studies indicate that fully differentiated memory T cells may TRAP more efficiently than early, activated T cells (data not shown), and it remains a question whether 'dysfunctional' T cells (that is, ones that lose the ability to produce cytokines or elicit cytotoxic activity upon T-cell antigen receptor triggering) will be able to TRAP APC membranes. Further studies should also include analysis of TRAP by CD4+ T cells. Preliminary analysis indicates that LCMV-specific CD4+ T cells acquire APC membranes and that heat-killed viral antigens can be used to sensitize APCs for recognition (data not shown). A subpopulation of virus-specific CD8+ T cells also become TRAP+ following exposure to APCs loaded with heat-killed viral antigens, and further studies are underway to determine the characteristics of these T-cell responses.
Here, we show evidence that memory T cells 'eat' APC membranes directly ex vivo in an antigen-specific manner. The purpose of this T-cell acquisition of APC proteins, however, is not entirely clear. It has been proposed to sustain T-cell antigen receptor triggering15 or to enable T cells to become 'nonprofessional' APCs. This nonprofessional presentation may function in a stimulatory capacity7 or as a mechanism for attenuating T-cell responses by inducing hyporesponsiveness14 or fratricide8. We propose another hypothesis: virus-specific memory T cells may 'cannibalize' APC membranes as a means of recycling cellular proteins, lipids or membranes from cells that are otherwise targeted for destruction. This would allow for more rapid T-cell proliferation to occur, as incorporation of pre-existing cellular factors into a dividing cell is likely to be more energy-efficient than synthesis of these factors strictly de novo. The ability to detect pathogen-specific T cells that have acquired APC membranes may prove useful both in quantification and in further elucidation of the biological and functional consequences of the process of APC membrane protein transfer.
Methods
Mice, cell lines and viral infection.
We purchased BALB/c mice from the Jackson Laboratory or they were bred at Oregon Health & Science University (OHSU). Mice 6–12 weeks old were infected intraperitoneally with 2 × 105 PFU of LCMV-Armstrong (53b) or 2 × 106 PFU of vaccinia-WR and were used at the indicated time points after infection. All experimental procedures were approved by the OHSU Institutional Animal Care and Use Committee. We maintained the A20 B-cell line in RPMI-1640 supplemented with 10% FBS (HyClone), L-glutamine and antibiotics, whereas we maintained J774 and BALBcl7 (BALB clone 7) cells in DMEM containing the same supplements. We infected A20 cells with live LCMV-Armstrong at a multiplicity of infection (MOI) of 0.5 for 22 h or with vaccinia-WR at an MOI of 10 for 15 h before use.
In vitro stimulation.
We washed infected and uninfected targets with PBS, and surface biotinylated them on ice for 20 min at a density of 107 per ml in biotinylation buffer (PBS, pH 7.4, plus 1 mM MgCl2 and 0.1 mM CaCl2) containing 1 mg/ml freshly added biotinylation reagent (EZ-Link Sulfo-NHS-LC biotin, 200 mg/ml in DMSO stored in single-use aliquots at –20 °C, Pierce). We then washed cells three times in RPMI supplemented with 5% FBS, glutamine and antibiotics (complete medium). For peptide coating of uninfected cells, we incubated biotinylated A20, J774, BALBcl7 or naive Thy1.1+ cells for 1 h at 37 °C at a density of 3 × 107 per ml in complete medium containing 10−7 M NP118 peptide. For TRAP assays performed with LCMV-immune C57BL/6 mice, we coated naive splenocytes with GP33, NP396 or GP276 using 10−6 M of the respective peptides. We washed cells twice in complete medium and once in PBS containing 1% FBS, and stained them with Pacific Blue–conjugated streptavidin (1:500 dilution, Molecular Probes) on ice for 30 min. We washed cells twice in PBS–1% FBS, then once in complete medium before use.
Single-cell suspensions of splenocytes isolated from naive or virus-infected mice were depleted of red cells by NH4Cl lysis. We used target cells to stimulate responder splenocytes in 96-well flat-bottom plates, with 5 × 105 of each cell type in 200 μl complete medium. We incubated cells at 37 °C, 6% CO2 for 6 h, with 2 μg/ml brefeldin A (Sigma) added for the final hour to allow quantification of IFN-γ production. Preliminary studies indicated that intact, viable target cells are required for APC membrane transfer to antigen-specific T cells; if infected or peptide-coated APCs are lysed by multiple freeze-thaw cycles before incubation with the T cells, then no appreciable TRAP+ T cells are identified (data not shown). In some experiments, we used IL-12 (R&D Systems) and IL-18 (Medical & Biological Laboratories) at a final concentration of 10 ng/ml each. For experiments involving naive splenocytes as APCs, we prepared target cells from Thy1.1+ mice, and we detected T cells from LCMV-immune Thy1.2+ mice by staining for Thy1.2+ T cells. To determine the sensitivity of the TRAP assay for detecting rare T-cell populations, we serially 1:2 diluted splenocytes from LCMV-immune mice with naive splenocytes for a combined total of 5 × 105 total splenocytes per well.
Staining and flow cytometry.
We blocked cells with antibody to Fc-γRIII/II (clone 2.4G2; 1.3 μg/ml) and mouse IgG (Sigma; 100 μg/ml) and stained them with antibody to CD8α (clone 5H10, CalTag), CD11a (clone 2D7, Pharmingen) and NP118-tetramer (NIH Tetramer Core Facility). We subsequently carried out intracellular staining with antibody to IFN-γ (clone XMG1.2, Pharmingen). For accurate quantification, we calculated the percentage of virus-specific T cells after subtracting background of nonspecific IFN-γ production or of APC membrane transfer following incubation with uncoated APCs. Forward- and side-scatter analysis by flow cytometry confirmed that APC membrane transfer was not the result of T-cell doublets or T cell–APC conjugates (Supplementary Fig. 3 online). Tetramers may be used in conjunction with other surface molecules such as CD44, CD62L or CD11a (LFA-1) to increase specificity and to provide further phenotypic information about T-cell populations. In our model, we found that costaining CD8+ T cells for CD11a improved the specificity of the TRAP assay; antigen-specific T cells uniformly fell within the CD11ahi CD8+ T-cell population. Pre-gating on CD11ahi T cells, however, is not absolutely required; effective quantification may be attained by simply staining for CD8+ T cells that have acquired APC membrane (Supplementary Fig. 1 online). We collected approximately 800,000 events on an LSRII flow cytometer (Becton Dickinson) and analyzed them with FlowJo software (Tree Star Inc.).
Statistical analysis.
We used linear regression analysis to compare T-cell quantification by IFN-γ ICCS versus the TRAP assay, and we used the paired Student's t-test to compare memory T-cell numbers quantified by NP118 peptide-coated APC versus LCMV-infected APCs (n = 9 mice from five experiments). We performed statistical analysis with Excel (Microsoft). A value of P < 0.05 was considered significant.
Note: Supplementary information is available on the Nature Medicine website.
References
Altman, J.D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996).
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Harrington, L.E., van der Most, R., Whitton, J.L. & Ahmed, R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J. Virol. 76, 3329–3337 (2002).
Manz, R., Assenmacher, M., Pfluger, E., Miltenyi, S. & Radbruch, A. Analysis and sorting of live cells according to secreted molecules, relocated to a cell-surface affinity matrix. Proc. Natl. Acad. Sci. USA 92, 1921–1925 (1995).
Liu, F. & Whitton, J.L. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J. Immunol. 174, 5936–5940 (2005).
Nepom, J.T., Benacerraf, B. & Germain, R.N. Acquisition of syngeneic I-A determinants by T cells proliferating in response to poly (Glu60Ala30Tyr10). J. Immunol. 127, 888–892 (1981).
Arnold, P.Y., Davidian, D.K. & Mannie, M.D. Antigen presentation by T cells: T cell receptor ligation promotes antigen acquisition from professional antigen-presenting cells. Eur. J. Immunol. 27, 3198–3205 (1997).
Huang, J.F. et al. TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science 286, 952–954 (1999).
Patel, D.M., Arnold, P.Y., White, G.A., Nardella, J.P. & Mannie, M.D. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J. Immunol. 163, 5201–5210 (1999).
Hudrisier, D., Riond, J., Mazarguil, H., Gairin, J.E. & Joly, E. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J. Immunol. 166, 3645–3649 (2001).
Hwang, I. et al. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J. Exp. Med. 191, 1137–1148 (2000).
Hudrisier, D. & Bongrand, P. Intercellular transfer of antigen-presenting cell determinants onto T cells: molecular mechanisms and biological significance. FASEB J. 16, 477–486 (2002).
Joly, E. & Hudrisier, D. What is trogocytosis and what is its purpose? Nat. Immunol. 4, 815 (2003).
Tsang, J.Y., Chai, J.G. & Lechler, R. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II:peptide complexes: another mechanism to limit clonal expansion? Blood 101, 2704–2710 (2003).
Wetzel, S.A., McKeithan, T.W. & Parker, D.C. Peptide-specific intercellular transfer of MHC class II to CD4+ T cells directly from the immunological synapse upon cellular dissociation. J. Immunol. 174, 80–89 (2005).
Tomaru, U. et al. Detection of virus-specific T cells and CD8+ T-cell epitopes by acquisition of peptide–HLA-GFP complexes: analysis of T-cell phenotype and function in chronic viral infections. Nat. Med. 9, 469–476 (2003).
Masopust, D., Vezys, V., Marzo, A.L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Belz, G.T., Xie, W. & Doherty, P.C. Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific CD8+ T cell responses. J. Immunol. 166, 4627–4633 (2001).
Hogan, R.J. et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 166, 1813–1822 (2001).
Chang, J. & Braciale, T.J. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 8, 54–60 (2002).
Johnson, A.J. et al. Prevalent class I-restricted T-cell response to the Theiler's virus epitope Db:VP2121–130 in the absence of endogenous CD4 help, tumor necrosis factor alpha, gamma interferon, perforin, or costimulation through CD28. J. Virol. 73, 3702–3708 (1999).
Marten, N.W., Stohlman, S.A., Zhou, J. & Bergmann, C.C. Kinetics of virus-specific CD8+-T-cell expansion and trafficking following central nervous system infection. J. Virol. 77, 2775–2778 (2003).
Slifka, M.K., Rodriguez, F. & Whitton, J.L. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature 401, 76–79 (1999).
Brosterhus, H. et al. Enrichment and detection of live antigen-specific CD4+ and CD8+ T cells based on cytokine secretion. Eur. J. Immunol. 29, 4053–4059 (1999).
Becker, C. et al. Adoptive tumor therapy with T lymphocytes enriched through an IFN- γ capture assay. Nat. Med. 7, 1159–1162 (2001).
Raue, H.P., Brien, J.D., Hammarlund, E. & Slifka, M.K. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J. Immunol. 173, 6873–6881 (2004).
Beadling, C. & Slifka, M.K. Differential regulation of virus-specific T-cell effector functions following activation by peptide or innate cytokines. Blood 105, 1179–1186 (2005).
Chattopadhyay, P.K., Yu, J. & Roederer, M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat. Med. 11, 1113–1117 (2005).
Wherry, E.J., Blattman, J.N., Murali-Krishna, K., van der Most, R. & Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003).
De Rosa, S.C. et al. Vaccination in humans generates broad T cell cytokine responses. J. Immunol. 173, 5372–5380 (2004).
Acknowledgements
NP118–H-2Ld-tetramer reagents were generously provided by the NIH Tetramer Core Facility in Atlanta, Georgia. This work was supported by National Institutes of Health grants AI054458 and AI051346 (to M.K.S.) and Oregon National Primate Research Center grant RR000163 (to M.K.S.).
Author information
Authors and Affiliations
Contributions
C.B. designed and performed the experiments and participated in writing the manuscript. M.K.S. designed experiments and participated in the data analysis and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
A variety of different fluorochromes can be used to visualize antigen-specific T cells with the TRAP assay. (PDF 614 kb)
Supplementary Fig. 2
Detection of T-cell responses to dominant and subdominant H-2b-restricted epitopes. (PDF 149 kb)
Supplementary Fig. 3
Size characteristics of virus-specific T cells and A20 targets. (PDF 489 kb)
Rights and permissions
About this article
Cite this article
Beadling, C., Slifka, M. Quantifying viable virus-specific T cells without a priori knowledge of fine epitope specificity. Nat Med 12, 1208–1212 (2006). https://doi.org/10.1038/nm1413
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1413
This article is cited by
-
Tumor cells endowed with professional antigen-presenting cell functions prime PBLs to generate antitumor CTLs
Journal of Molecular Medicine (2019)
-
Intercellular Exchange of Surface Molecules and its Physiological Relevance
Archivum Immunologiae et Therapiae Experimentalis (2010)
-
A simple trogocytosis-based method to detect, quantify, characterize and purify antigen-specific live lymphocytes by flow cytometry, via their capture of membrane fragments from antigen-presenting cells
Nature Protocols (2006)