Abstract
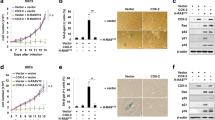
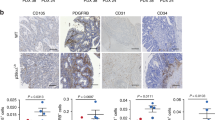
The finding of frequent nitric oxide synthase expression in human cancers indicates that nitric oxide has a pathophysiological role in carcinogenesis. To determine the role of nitric oxide in tumor progression, we generated human carcinoma cell lines that produced nitric oxide constitutively. Cancer cells expressing inducible nitric oxide synthase that had wild-type p53 had reduced tumor growth in athymic nude mice, whereas those with mutated p53 had accelerated tumor growth associated with increased vascular endothelial growth factor expression and neovascularization. Our data indicate that tumor-associated nitric oxide production may promote cancer progression by providing a selective growth advantage to tumor cells with mutant p53, and that inhibitors of inducible nitric oxide synthase may have therapeutic activity in these tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Thomsen, L.L. et al. Nitric oxide synthase activity in human breast cancer. Br. J. Cancer 72, 41– 44 (1995).
Ellie, E., Loiseau, H., Lafond, F., Arsaut, J. & Demotes-Mainard, J. Differential expression of inducible nitric oxide synthase mRNA in human brain tumors. Neuroreport 7, 294–296 (1995).
Ambs, S. et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 58, 334–341 (1998).
Gallo, O. et al. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J. Natl. Cancer Inst. 90, 587–596 (1998).
Thomsen, L.L. et al. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 57, 3300–3304 (1997).
Melillo, G. et al. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J. Exp. Med. 182, 1683–1693 (1995).
Ziche, M. et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor—induced but not basic fibroblast growth factor—induced angiogenesis. J. Clin. Invest. 99, 2625– 2634 (1997).
Chin, K. et al. Induction of vascular endothelial growth factor by nitric oxide in human glioblastoma and hepatocellular carcinoma cells. Oncogene 15, 437–442 (1997).
Jenkins, D.C. et al. Roles of nitric oxide in tumor growth. Proc. Natl. Acad. Sci. USA 92, 4392–4396 (1995).
Edwards, P. et al. Tumor cell nitric oxide inhibits cell growth in vitro, but stimulates tumorigenesis and experimental lung metastasis in vivo. J. Surg. Res. 63, 49–52 (1996).
Garcia-Cardena, G. & Folkman, J. Is there a role for nitric oxide in tumor angiogenesis? J. Natl. Cancer Inst. 90, 560–561 (1998).
Dong, Z., Staroselsky, A.H., Qi, X., Xie, K. & Fidler, I.J. Inverse correlation between expression of inducible nitric oxide synthase activity and production of metastasis in K-1735 murine melanoma cells. Cancer Res. 54, 789–793 (1994).
Xie, K. et al. Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. J. Exp. Med. 181, 1333–1343 (1995).
Geng, Y.J., Hellstrand, K., Wennmalm, A. & Hansson, G.K. Apoptotic death of human leukemic cells induced by vascular cells expressing nitric oxide synthase in response to gamma-interferon and tumor necrosis factor-alpha. Cancer Res. 56, 866–874 (1996).
Nicotera, P., Bonfoco, E. & Brune, B. Mechanisms for nitric oxide-induced cell death: involvement of apoptosis. Adv. Neuroimmunol. 5, 411– 420 (1997).
Kim, Y.M., Talanian, R.V. & Billiar, T.R. Nitric oxide inhibits apoptosis . J. Biol. Chem. 272, 1402–1411 (1997).
Mannick, J.B., Miao, X.Q. & Stamler, J.S. Nitric oxide inhibits Fas-induced apoptosis. J. Biol. Chem. 272, 24125–24128 (1997).
Messmer, U.K. & Brune, B. Nitric oxide-induced apoptosis: p53-dependent and p53-independent signalling pathways. Biochem. J. 319, 299–305 (1996).
Forrester, K. et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase (NOS2) expression by wild-type p53. Proc. Natl. Acad. Sci. USA 93, 2442–2447 (1996).
Tzeng, E., Billiar, T.R., Robbins, P.D., Loftus, M. & Stuehr, D.J. Expression of human inducible NO synthase in a tetrahydrobiopterin promotes assembly of enzyme subunits into an active dimer. Proc. Natl. Acad. Sci. USA 92, 11771–11775 (1995).
Lewis, R.S., Tamir, S., Tannenbaum, S.R. & Deen, W.M. Kinetic analysis of the fate of nitric oxide synthesized by macrophages in vitro. J. Biol. Chem. 270, 29350– 29355 (1995).
Polyak, K., Waldman, T., He, T.C., Kinzler, K.W. & Vogelstein, B. Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 10, 1945– 1952 (1996).
Griffiths, M.J., Messent, M., MacAllister, R.J. & Evans, T.W. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br. J. Pharmacol. 110, 963–968 (1993).
Vermeulen, P.B. et al. Correlation of intratumoral microvessel density and p53 protein overexpression in human colorectal adenocarcinoma. Microvasc. Res. 51, 164–174 (1996).
Hentze, M.W. & Kuhn, L.C. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc. Natl. Acad. Sci. USA 93, 8175–8182 (1996).
Gleadle, J.M., Ebert, B.L., Firth, J.D. & Ratcliffe, P.J. Regulation of angiogenic growth factor expression by hypoxia, transition metals, and chelating agents. Am. J. Physiol. 268, C1362–8 (1995).
Piazza, G.A. et al. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 55, 3110–3116 (1995).
Tsurumi, Y. et al. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nature Med. 3, 879–886 (1997).
Dameron, K.M., Volpert, O.V., Tainsky, M.A. & Bouck, N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science 265, 1582–1584 (1994).
Greenblatt, M.S., Bennett, W.P., Hollstein, M. & Harris, C.C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 54, 4855– 4878 (1994).
Ambs, S., Hussain, S.P. & Harris, C.C. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J. 11, 443–448 (1997).
Geller, D.A. et al. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc. Natl. Acad. Sci. USA 90, 3491–3495 (1993).
Rak, J. et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 55, 4575–4580 (1995).
Acknowledgements
We thank D. Dudek for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ambs, S., Merriam, W., Ogunfusika, M. et al. p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nat Med 4, 1371–1376 (1998). https://doi.org/10.1038/3957
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/3957
This article is cited by
-
Phase I studies of vorinostat with ixazomib or pazopanib imply a role of antiangiogenesis-based therapy for TP53 mutant malignancies
Scientific Reports (2020)
-
An in vitro study ascertaining the role of H2O2 and glucose oxidase in modulation of antioxidant potential and cancer cell survival mechanisms in glioblastoma U-87 MG cells
Metabolic Brain Disease (2017)
-
Antitumoral gene-based strategy involving nitric oxide synthase type III overexpression in hepatocellular carcinoma
Gene Therapy (2016)
-
Rhus coriaria suppresses angiogenesis, metastasis and tumor growth of breast cancer through inhibition of STAT3, NFκB and nitric oxide pathways
Scientific Reports (2016)
-
The homeoprotein DLX4 controls inducible nitric oxide synthase-mediated angiogenesis in ovarian cancer
Molecular Cancer (2015)