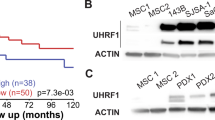
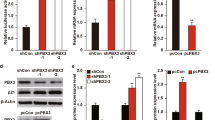
Abstract
Paxillin (PXN), a key component of the focal adhesion complex, has been associated with cancer progression, but the underlying mechanisms are poorly understood. The purpose of this study was to elucidate mechanisms by which PXN affects cancer growth and progression, which we addressed using cancer patient data, cell lines, and orthotopic mouse models. We demonstrated a previously unrecognized mechanism whereby nuclear PXN enhances angiogenesis by transcriptionally regulating SRC expression. SRC, in turn, increases PLAT expression through NF-ĸB activation; PLAT promotes angiogenesis via LRP1 in endothelial cells. PXN silencing in ovarian cancer mouse models reduced angiogenesis, tumor growth, and metastasis. These findings provide a new understanding of the role of PXN in regulating tumor angiogenesis and growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zhou J, Yi Q, Tang L. The roles of nuclear focal adhesion kinase (FAK) on cancer: a focused review. J Exp Clin Cancer Res. 2019;38:250.
Panetti TS. Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front Biosci: a J virtual Libr. 2002;7:d143–150.
McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer—a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–15.
Li BZ, Lei W, Zhang CY, Zhou F, Li N, Shi SS, et al. Increased expression of paxillin is found in human oesophageal squamous cell carcinoma: a tissue microarray study. J Int Med Res. 2008;36:273–8.
Mackinnon AC, Tretiakova M, Henderson L, Mehta RG, Yan BC, Joseph L, et al. Paxillin expression and amplification in early lung lesions of high-risk patients, lung adenocarcinoma and metastatic disease. J Clin Pathol. 2011;64:16–24.
Shi J, Wang S, Zhao E, Shi L, Xu X, Fang M. Paxillin expression levels are correlated with clinical stage and metastasis in salivary adenoid cystic carcinoma. J Oral Pathol Med. 2010;39:548–51.
Deakin NO, Pignatelli J, Turner CE. Diverse roles for the paxillin family of proteins in cancer. Genes Cancer. 2012;3:362–70.
López-Colomé AM, Lee-Rivera I, Benavides-Hidalgo R, López E. Paxillin: a crossroad in pathological cell migration. J Hematol Oncol. 2017;10:50.
Kenny HA, Chiang C-Y, White EA, Schryver EM, Habis M, Romero IL, et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Investig. 2014;124:4614–28.
Ip CK, Yung S, Chan TM, Tsao SW, Wong AS. p70 S6 kinase drives ovarian cancer metastasis through multicellular spheroid-peritoneum interaction and P-cadherin/b1 integrin signaling activation. Oncotarget. 2014;5:9133–49.
Chen DL, Wang ZQ, Ren C, Zeng ZL, Wang DS, Luo HY, et al. Abnormal expression of paxillin correlates with tumor progression and poor survival in patients with gastric cancer. J Transl Med. 2013;11:277.
Kratimenos P, Koutroulis I, Syriopoulou V, Michailidi C, Delivoria-Papadopoulos M, Klijanienko J, et al. FAK-Src-paxillin system expression and disease outcome in human neuroblastoma. Pediatr Hematol Oncol. 2017;34:221–30.
Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–39.
Dong JM, Lau LS, Ng YW, Lim L, Manser E. Paxillin nuclear-cytoplasmic localization is regulated by phosphorylation of the LD4 motif: evidence that nuclear paxillin promotes cell proliferation. Biochem J. 2009;418:173–84.
Sen A, De Castro I, Defranco DB, Deng FM, Melamed J, Kapur P, et al. Paxillin mediates extranuclear and intranuclear signaling in prostate cancer proliferation. J Clin Investig. 2012;122:2469–81.
Sanchez-Garcia I, Rabbitts TH. The LIM domain: a new structural motif found in zinc-finger-like proteins. Trends Genet: TIG. 1994;10:315–20.
Hou XY, Liu Y, Zhang GY. PP2, a potent inhibitor of Src family kinases, protects against hippocampal CA1 pyramidal cell death after transient global brain ischemia. Neurosci Lett. 2007;420:235–9.
Ulfhammer E, Larsson P, Karlsson L, Hrafnkelsdóttir T, Bokarewa M, Tarkowski A, et al. TNF-α mediated suppression of tissue type plasminogen activator expression in vascular endothelial cells is NF-κB- and p38 MAPK-dependent. J Thrombosis Haemost. 2006;4:1781–9.
Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Res. 2007;67:7368–77.
Landen CN Jr., Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–8.
Hu K, Lin L, Tan X, Yang J, Bu G, Mars WM, et al. tPA protects renal interstitial fibroblasts and myofibroblasts from apoptosis. J Am Soc Nephrology. 2008;19:503–14.
Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–24.
Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000;100:293–6.
Phung CD, Tran TH, Pham LM, Nguyen HT, Jeong J-H, Yong CS, et al. Current developments in nanotechnology for improved cancer treatment, focusing on tumor hypoxia. J Controlled Release. 2020;324:413–29.
Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–46.
Byers LA, Sen B, Saigal B, Diao L, Wang J, Nanjundan M, et al. Reciprocal regulation of c-Src and STAT3 in non-small cell lung cancer. Clinical cancer research: an official journal of the American Association for. Cancer Res. 2009;15:6852–61.
Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–95.
Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, et al. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22:901–15.
Diaz VM, Planaguma J, Thomson TM, Reventos J, Paciucci R. Tissue plasminogen activator is required for the growth, invasion, and angiogenesis of pancreatic tumor cells. Gastroenterology. 2002;122:806–19.
Ohki M, Ohki Y, Ishihara M, Nishida C, Tashiro Y, Akiyama H, et al. Tissue type plasminogen activator regulates myeloid-cell dependent neoangiogenesis during tissue regeneration. Blood. 2010;115:4302–12.
Hu W, Liu T, Ivan C, Sun Y, Huang J, Mangala LS, et al. Notch3 pathway alterations in ovarian cancer. Cancer Res. 2014;74:3282–93.
Noh KH, Kang HM, Yoo W, Min Y, Kim D, Kim M, et al. Ubiquitination of PPAR-gamma by pVHL inhibits ACLY expression and lipid metabolism, is implicated in tumor progression. Metabolism. 2020;110:154302.
Rupaimoole R, Wu SY, Pradeep S, Ivan C, Pecot CV, Gharpure KM, et al. Hypoxia-mediated downregulation of miRNA biogenesis promotes tumour progression. Nat Comm. 2014;5:5202.
Wu SY, Rupaimoole R, Shen F, Pradeep S, Pecot CV, Ivan C, et al. A miR-192-EGR1-HOXB9 regulatory network controls the angiogenic switch in cancer. Nat Comm. 2016;7:11169.
Acknowledgements
Portions of this work were supported by the National Institutes of Health (P30 CA016672, P50 CA217685, P50 CA098258, and R35 CA209904), the Blanton-Davis Ovarian Cancer Research Program, American Cancer Society Research Professor Award, Judy’s Mission, and the Frank McGraw Memorial Chair in Cancer Research. SP is supported by the Ovarian Cancer Research Fund Alliance (OCRFA). ES is supported by Ovarian Cancer Research Alliance (OCRA number FP00006137). KHN is supported by the KRIBB Research Initiative Program. We thank Dr. Bryan F. Tutt (Department of Scientific Publications) and Nicholas B. Jennings for kindly reviewing this manuscript.
Author information
Authors and Affiliations
Contributions
KHN, D-HB, H-JC, and MSK designed the experiments. KHN, D-HB, and H-JC performed the experiments, analyzed data, and wrote the manuscript; SYW, MSK, SP, IC, M-SC, RR, CR-A, RAP, SKD, ES, and LSM performed experiments and analyzed data. GLB discussed and contributed to data. AKS supervised the entire project, designed the experiments, analyzed data, and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AKS (consulting for Kiyatec and Merck; research funding from M-Trap; stockholder in BioPath).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noh, K., Bach, DH., Choi, HJ. et al. The hidden role of paxillin: localization to nucleus promotes tumor angiogenesis. Oncogene 40, 384–395 (2021). https://doi.org/10.1038/s41388-020-01517-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01517-3
This article is cited by
-
Mapping the distribution of tension across paxillin upon shear stress with FRET-based biosensor
Med-X (2024)
-
Prognostic implications of PPL expression in ovarian cancer
Discover Oncology (2022)