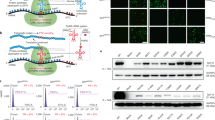
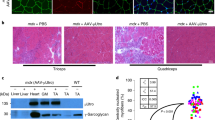
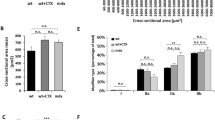
Abstract
Limb-girdle muscular dystrophies 2C–F represent a family of autosomal recessive diseases caused by defects in sarcoglycan genes1. The cardiomyopathic hamster is a naturally occurring model for limb-girdle muscular dystrophy caused by a primary deficiency in δ-sarcoglycan2,3,4,5. We show here that acute sarcolemmal disruption occurs in this animal model during forceful muscle contraction. A recombinant adeno-associated virus vector encoding human δ-sarcoglycan conferred efficient and stable genetic reconstitution in the adult cardiomyopathic hamster when injected directly into muscle. A quantitative assay demonstrated that vector-transduced muscle fibers are stably protected from sarcolemmal disruption; there was no associated inflammation or immunologic response to the vector-encoded protein. Efficient gene transduction with rescue of the sarcoglycan complex in muscle fibers of the distal hindlimb was also obtained after infusion of recombinant adeno-associated virus into the femoral artery in conjunction with histamine-induced endothelial permeabilization. This study provides a strong rationale for the development of gene therapy for limb-girdle muscular dystrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bonnemann, C., McNally, E. & Kunkel, L. Beyond dystrophin: current progress in the muscular dystrophies. Curr. Opin. Pediatr. 8, 569 –582 (1996).
Homburger, F., Baker, J., Nixon, C. & Whitney, R. Primary, generalized polymyopathy and necrosis in an inbred line of Syrian hamsters. Med. Exp. 6, 339–345 ( 1962).
Roberds, S. et al. Disruption of the dystrophin-glycoprotein complex in the cardiomyopathyic hamster. J. Biol. Chem. 268, 11496– 11499 (1993).
Nigro, V. et al. Identification of the Syrian hamster cardiomyopathy gene. Hum. Mol. Genet. 6, 601–607 (1997).
Sakamoto, A. et al. Both hypertrophic and dilated cardiomyopathies are caused by mutation of the same gene, delta sarcoglycan, in hamster: an animal model of disrupted dystrophin-associated glycoprotein complex. Proc. Natl. Acad. Sci. USA 94, 13873–13878 (1997).
Worton, R. Muscular dystrophies: diseases of the dystrophin-glycoprotein complex. Science 270, 755–6 ( 1995).
Fisher, K. et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nature Med. 3, 306–312 (1997).
Homburger, F., Baker, J., Wilgram, G., Caulfield, J. & Nixon, C. Hereditary dystrophy-like myopathy. The histopathology of hereditary dystrophy-like myopathy in Syrian hamsters. Arch. Pathol. 81, 302–7 ( 1966).
Cox, G. et al. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature 364, 725–29 (1993).
Ragot, T. et al. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature 361, 647–650 (1993).
Holt, K. et al. Functional rescue of the sarcoglycan complex in the BIO 14.6 hamster using delta-sarcoglygan gene transfer. Mol. Cell 1, 841–848 (1998).
Cox, G.A., Sunada, Y., Campbell, K.P. & Chamberlain, J.S. Dp71 can restore the dystrophin-associated glycoprotein complex in muscle but fails to prevent dystrophy. Nature Genet. 8, 333–339 (1994).
Petrof, B.J., Shrager, J.B., Stedman, H.H., Kelly, A.M. & Sweeney, H.L. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. 90, 3710–14 ( 1993).
Straub, V., Rafael, J., Chamberlain, J. & Campbell, K. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 139, 375– 385 (1997).
Deconinck, N., Ragot, T., Marechal, G., Perricaudet, M. & Gillis, J. Functional protection of dystrophic mouse (mdx) muscles after adenovirus-mediated transfer of a dystrophin minigene. Proc. Natl. Acad. Sci. USA 93, 3570–3574 (1996).
DeMatteo, R., Chu, G., Chang, E., Barker, C. & Markmann, J. Long-lasting adenovirus transgene expression in mice through neonatal intrathymic tolerance induction without the use of immunosuppression. J. Virol. 71, 5330–5 (1997).
Nielsen, H. Histamine-2 receptor antagonists as immunomodulators: new therapeutic views? Ann. Med. 28, 107–13 (1996).
Stedman, H.H. et al. Nebulin cDNAs detect a 25-kilobase transcript in skeletal muscle and localize to human chromosome 2. Genomics 2, 1–7 (1988).
Samulski, R., Chang, L. & Shenk, T. A recombinant plasmid–from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 61, 3096-3101 (1987).
Sanes, J., Rubenstein, J. & Nicolas, J. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 5, 3133–3142 (1986).
Brumback, R.A. & and Leech, R.W. in Color Atlas of Muscle Histochemistry, 13;21 (PSG, Littleton, Massachusetts, 1984).
Acknowledgements
Acknowledgments These studies were supported in part by grants from the National Institutes of Health (5PO1AR/NS43648) and the Muscular Dystrophy Association. L.S. is a research fellow of the American Heart Association. S.K. is a research fellow of the Association Francaise contre les Myopathies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greelish, J., Su, L., Lankford, E. et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med 5, 439–443 (1999). https://doi.org/10.1038/7439
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/7439
This article is cited by
-
In vivo restoration of dystrophin expression in mdx mice using intra-muscular and intra-arterial injections of hydrogel microsphere carriers of exon skipping antisense oligonucleotides
Cell Death & Disease (2022)
-
A Needleless Liquid Jet Injection Delivery Method for Cardiac Gene Therapy: a Comparative Evaluation Versus Standard Routes of Delivery Reveals Enhanced Therapeutic Retention and Cardiac Specific Gene Expression
Journal of Cardiovascular Translational Research (2014)
-
Therapy of Genetic Disorders: Novel Therapies for Duchenne Muscular Dystrophy
Current Pediatrics Reports (2014)
-
Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution
Gene Therapy (2011)
-
A muscle-targeting peptide displayed on AAV2 improves muscle tropism on systemic delivery
Gene Therapy (2009)