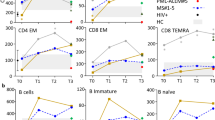
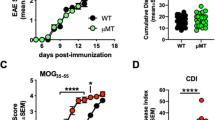
Abstract
Demyelination alone has been considered sufficient for development of neurological deficits following central nervous system (CNS) disease. However, extensive demyelination is not always associated with clinical deficits in patients with multiple sclerosis (MS), the most common primary demyelinating disease in humans. We used the Theiler's murine encephalomyelitis virus model of demyelination to investigate the role of major histocompatibility complex (MHC) class I and class II gene products In the development of functional and neurophysiological deficits following demyelination. We measured spontaneous clinical activity by two independent assays and recorded hind-limb motor-evoked potentials in infected class I-deficient and class 11-deficient mice of an identical genetic background as well as in highly susceptible SJL/J mice. The results show that despite a similar distribution and extent of demyelinated lesions in all mice, only class I-deficient mice were functionally normal. We propose that the mechanism by which demyelinated class I-deficient mice maintain neurologic function results from increased sodium channel densities and the relative preservation of axons. These findings are the first to implicate a role for MHC class I in the development of neurological deficits following demyelination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rodriguez, M., Oleszak, E. & Leibowitz, J. Theiler's murine encephalomyelitis: A model of demyelination and persistence of virus. Crit. Rev. Immunol. 7, 325–365 (1987).
Fiette, L., Aubert, C., Brahic, M. & Rossi, C.P. Theiler's virus infection of β2-mi-croglobulin-deficient mice. J. Virol. 67, 589–592 (1993).
Pullen, L.C., Miller, S.D., Dal Canto, M.C. & Kim, B.S. Class I-deficient resistant mice intracerebrally inoculated with Theiler's virus show an increased T cell response to viral antigens and susceptibility to demyelination. Eur. J. Immunol. 23, 2287–2293 (1993).
Rodriguez, M., et al. Abrogation of resistance to Theiler's-induced demyelination in H–2b mice deficient in β2-microglobulin. J. Immunol. 151, 266–276 (1993).
Njenga, M.K., et al. Theiler's virus persistence and demyelination in major histo-compatibility complex class II-deficient mice. J. Virol. 70, 1729–1737 (1996).
Rodriguez, M., Leibowitz, J. & David, C.S. Susceptibility to Theiler's virus-induced demyelination: Mapping of the gene within the H–2D region. J. Exp. Med. 163, 620–631 (1986).
Rodriguez, M. & David, C.S. Demyelination induced by Theiler's virus: Influence of the H–2 haplotype. J. Immunol. 135, 2145–2148 (1985).
Foster, R.E., Whalen, C.C. & Waxman, S.G. Reorganization of the axon membrane in demyelinated peripheral nerve fibers: Morphological evidence. Science 210, 661–663 (1980).
Bostock, H., Hall, S.M. & Smith, K.J. Demyelinated axons can form ‘nodes’ prior to remyelination.). Physiol. (Lond.) 308, 21P–23P (1980).
Smith, K.J., Bostock, H. & Hall, S.M. Saltatory conduction precedes remyelination in axons demyelinated with lysophosphatidyl choline. J. Neurol. Sci. 54, 13–31 (1982).
Waxman, S.G. Demyelination in spinal cord injury and multiple sclerosis: What can we do to enhance functional recovery? J. Neurotrauma 9, S105–S117 (1992).
Rodriguez, M. & Sriram, S. Successful therapy of Theiler's virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J. Immunol. 140, 2950–2955 (1988).
Miller, D.J., Rivera-Quiñones, C., Njenga, M.K., Leibowitz, J. & Rodriguez, M. Spontaneous CNS remyelination in p2 microglobulin-deficient mice following virus-induced demyelination. J. Neurosci. 15, 8345–8352 (1995).
Miller, D.J., Njenga, M.K., Murray, P.D., Leibowitz, J. & Rodriguez, M. A monoclonal natural autoantibody that promotes remyelination suppresses central nervous system inflammation and increases virus expression after Theiler's virus-induced demyelination. Int. Immunol. 8, 131–141 (1996).
Brinkmeier, H., Kaspar, A., Wietholter, H. & Rudel, R. Interleukin-2 inhibits sodium currents in human muscle cells. Pfluegers Arch. 420, 621–623 (19??).
Black, J.A., Felts, P., Smith, K.J., Kocksis, J.D. & Waxman, S.C. Distribution of sodium channels in chronically demyelinated spinal cord axons: Immuno-structural localization and electrophysiological observations. Brain Res. 544, 59–70 (1991).
McDonald, W.I. & Sears, T.A. Effect of demyelination on conduction in the central nervous system. Brain 93, 583–598 (1970).
Filippi, M., et al. Correlations between changes in disability and T2-weighted brain MRI activity in multiple sclerosis: A follow-up study. Neurology 45, 255–260 (1995).
Gilbert, J.J. & Sadler, M. Unsuspected multiple sclerosis. Arch. Neurol. 40, 533–536 (1983).
Mackay, R.P. & Hirano, A. Forms of benign multiple sclerosis: Report of two “clinically silent” cases discovered at autopsy. Arch. Neurol. 17, 588–600 (1967).
Moll, C., Mourre, C., Lazdunsky, M. & Ulrich, J. Increase of sodium channels in demyelinated lesions of multiple sclerosis. Brain Res. 556, 311–316 (1991).
Hauser, S.L., et al. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann. Neurol. 19, 578–587 (1986).
Sibley, W.A., Bamford, C.R. & Clark, K. Clinical viral infections and multiple sclerosis. Lancet 1, 1313–1315 (1985).
The IFNβ Multiple Sclerosis Group. Interferon beta-1 b is effective in relapsing-re-mitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double blind, placebo-controlled trial. Neurology 43, 655–661 (1993).
Iuliano, B.A., Schmelzer, J.D., Thiemann, R.L., Low, P.A. & Rodriguez, M. Motor and somatosensory-evoked potentials in mice infected with Theiler's murine encephalomyelitis virus. J. Neurol. Sci. 123, 186–194 (1994).
Dugandzija-Novakovic, S., Koszowski, A.G., Levinson, S.R. & Shrager, P. Clustering of Na+ channels and node of Ranvier formation in remyelinating axons. J. Neurosci. 15, 492–503 (1995).
Mourre, C., Widmann, C. & Lazdunski, M. Saxitoxin-sensitive Na+ channels: Presynaptic localization in cerebellum and hippocampus of neurological mutant mice. Brain Res. 533, 196–202 (1990).
Robb, R.A., et al. ANALYZE: A comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput. Med. Imag. Graphics 13, 433–454 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rivera-Quiñones, C., McGavern, D., Schmelzer, J. et al. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat Med 4, 187–193 (1998). https://doi.org/10.1038/nm0298-187
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0298-187
This article is cited by
-
Multiple sclerosis: experimental models and reality
Acta Neuropathologica (2017)
-
Major histocompatibility complex class I molecules protect motor neurons from astrocyte-induced toxicity in amyotrophic lateral sclerosis
Nature Medicine (2016)
-
Animal models of multiple sclerosis: the good, the bad and the bottom line
Nature Neuroscience (2012)
-
Neuropathogenesis of Theiler’s Murine Encephalomyelitis Virus Infection, An Animal Model for Multiple Sclerosis
Journal of Neuroimmune Pharmacology (2010)
-
Pathophysiological aspects of the formation of neurological deficit in multiple sclerosis
Neuroscience and Behavioral Physiology (2009)