Abstract
Therapeutic success of B cell-targeting approaches in multiple sclerosis (MS) has intensified research into the pathogenic and regulatory roles these cells play in demyelinating disease. Dissecting the function of B cells in the MS mouse model experimental autoimmune encephalomyelitis (EAE) is largely confined to induction with either the myelin oligodendrocyte glycoprotein epitope MOG35–55 or the full-length recombinant human MOG protein, the latter representing the most-used B cell-dependent EAE model. There is a clear need to investigate B cell function in additional myelin antigen contexts. Unlike MOG35–55, where lack of B cells yields more severe disease, we show here that the immunodominant myelin proteolipid protein epitope (PLP178–191) elicited identical EAE in WT and μMT mice, suggesting an absence of B cell engagement by this peptide. We hypothesized that a longer PLP antigen may better engage B cells and designed a peptide encompassing the extracellular domains (ECD) of PLP. We demonstrate here that PLPECD-immunized B cell-deficient mice failed to exhibit EAE. In contrast, PLPECD induced EAE not only in WT mice, but in B cell-sufficient mice incapable of secreting antibodies, suggesting a predominant antigen presentation role. These results establish a novel, efficient B cell-dependent EAE model.
Similar content being viewed by others
Introduction
Recent clinical trials involving the targeted deletion of B cells1,2,3 have reinvigorated intense interest in the role of this lymphocyte in immune-mediated demyelinating diseases such as multiple sclerosis (MS). It is essential to have multiple models, whereby various angles of B cell biology (such as their potential pathogenic or regulatory roles) can be deciphered in the context of disease. Historically, myelin oligodendrocyte glycoprotein (MOG) sequence 35–55 has been used to great success in inducing demyelinating disease in WT B6 mice4,5, where knockout capabilities and genetic tools like the 2D2 system (mice harboring a TCR transgene specific for the MOG35–55 epitope) are available6.
B cell studies in MOG-induced demyelinating disease have led to a contradictory literature, in that there is evidence for both pathogenic and regulatory roles, as well as models that require and yet do not require the presence of B cells for EAE. These discrepancies can largely be explained by differences in model type, animal strain, and inciting antigen used. For instance, antibody titers and B cell numbers in the cerebrospinal fluid (CSF) have been shown to correlate with disease severity in mice and humans7,8,9,10. Likewise, full length recombinant human MOG (hMOG)-reactive serum transfers from B6 mice have been shown to cause disease in healthy animals11. Decreased demyelination in B cell-deficient versus WT B6 mice has been observed (yet both exhibited disease)12 while other data demonstrate that induction of EAE failed altogether in B cell-deficient mice13. Interestingly, these studies utilized rodent MOG versus human MOG, respectively. It has further been demonstrated that deleting B cells from MOG35–55 EAE mice during peak disease ameliorates clinical symptoms14. In addition to pathogenic antibody studies, B cells have been shown to contribute to rodent MOG-induced EAE in C3H mice as well as hMOG-induced EAE in B6 mice by reactivating CD4 T cells in the CNS through a likely antigen presentation function15,16,17,18. These reports of B cell pathogenicity notwithstanding, it has also been demonstrated that B cell-deficient mice cannot recover from myelin basic protein-induced EAE disease in B10.PL mice19 and have a quicker and more robust MOG35–55 and rodent MOG disease onset compared to their WT B6 counterparts20,21. Further, deletion of B cells prior to MOG35–55 disease onset led to exacerbated disease14, adding to studies attributing regulatory function to B cells during EAE.
These data clearly suggest that B cell subsets have varying functional capacities (IL-10-producing Bregs20 versus CD4 T cell-reactivating CNS B cells15, for example). However, the general discrepancies seen in these reports, particularly regarding experiments involving B cell-deficient mice, can be explained by differences in the length of MOG antigen used for immunization (short peptide versus full-length recombinant protein), and the origin of MOG antigen (rodent versus human). It turns out that a single amino acid at position 42 (serine in the rodent, proline in the human) in the MOG sequence22,23 can have striking consequences for the role of B cells in MOG-induced EAE models. Ultimately, it is understood that recombinant hMOG is necessary to induce a robust B cell-dependent mouse model of MS13,22,23. Yet it is still unclear whether this is due to pathogenic antibody production or requirement for antigen processing and presentation. In addition, production of the hMOG protein is cumbersome and represents a significant rate-limiting step in the study of B cell functionality in vivo during demyelinating disease. Finally, heavy reliance on a single model may bias our understanding of the role of these cells in complex human disease.
Investigating the role of B cells in myelin proteolipid protein (PLP)-induced EAE models is needed. PLP is highly conserved, where complete amino acid sequence homology is shared between mice and humans, and is very abundant in the CNS, comprising 50% of total myelin protein24. Indeed, understanding immune responses in this context is extremely relevant and may provide insights into the pathogenicity and regulation of MS. Whereas PLP-targeted responses are a focus in the relapsing-remitting SJL mouse model of EAE25,26, this protein or its peptides are underutilized in the context of B6 models, where there is a greater availability of genetic tools to dissect the function of various cells and molecules. We have recently utilized PLP178–191-induced EAE in B6 mice to demonstrate the robust disease regulatory role of PLP178–191-induced CD8 T cell responses27,28,29,30. We therefore decided to focus on PLP-induced EAE to develop a model wherein B cell function could also be delineated. Interestingly, our findings here suggest that B cells are ancillary during the immunodominant PLP178–191-incduced form of EAE in B6 mice. We hypothesized that designing a longer peptide may provide a processable antigen for B cells to engage and present to T cells, thus alleviating the need for intricate whole protein production methodology. Here, we utilized a novel designed peptide (PLPECD, an 83-mer that incorporates the extracellular sequences of PLP while excluding positions buried within or just proximal to transmembrane regions) to test B cell-dependency in the context of PLP-induced demyelinating disease in B6 mice. We offer an efficient new mouse model suitable to investigating potential roles for B cells in EAE.
Materials and methods
Mice
C57BL/6 J and μMT mice were purchased from Jackson Laboratories (Bar Harbor, ME). AID−/−μS−/− mice were kindly provided by Drs. Frances Lund and Troy Randall (University of Alabama, Birmingham) and Dr. Tasuku Honjo (Kyoto University, Japan). All mice were kept in barrier rooms at the University of Iowa Animal Care Facility under 12-hour light/dark cycle, fed ad libitum, and humanely cared for and studied as approved by the University of Iowa’s Institutional Animal Care and Use Committee and in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Peptides
Myelin peptides PLP178–191 (NTWTTCQSIAFPSK), MOG35–55 (MEVGWYRSPFSRVVHLYRNGK), and control peptide OVA323–339 (ISQAVHAAHAEINEAGR) were purchased from GenScript (Piscataway, NJ). Myelin peptide PLP36–58;179–238 or “PLPECD” (HEALTGTEKLIETYFSKNYQDYETWTTCQSI AFPSKTSASIGSLCADARMYGVLPWNAFPGKVCGSNLLSICKTAEFQMTFHL) was purchased from Thermo Pierce Custom Peptides (Waltham, MA).
EAE immunization and scoring
As described previously27,28,29,30, mice were immunized s.c. on d0 in the right and left flank with 100ug of PLP antigen emulsified 1:1 volume in complete Freund’s adjuvant supplemented with 4 mg/ml Mycobacterium tuberculosis (CFA; Becton Dickinson, Franklin Lakes, NJ), followed by 250 ng pertussis toxin (PTx; List Biologicals, Campbell, CA) i.p. on days 0 and 2. Clinical scores were recorded in a blinded manner by ascending hind limb paralysis: 0, no symptoms; 1, loss of tail tonicity; 2, partial hind limb weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis; 5, moribund or death.
Delayed-type hypersensitivity (DTH)/ear swelling assays
As described previously30, ear pinnae of briefly anesthetized (isoflurane USP, Clipper Distributing, St. Joseph, MO) ear pinnae of mice were injected with 150ug PLP178–191, PLPECD, or OVA323–339 in 15 µl volume PBS at the indicated timepoints post-immunization with a 30 G needle from a 1cc syringe. Additional control ears received 15 µl PBS alone. Delta ear swelling was measured in a blinded manner with an engineer’s micrometer (Mitutoyo USA, Aurora, IL) and calculated by ear thickness (mm) at 48 h minus thickness at 0 h.
Statistical analysis
When comparing two groups, data were analyzed using the Welch’s t-test. For multiparametric data, the two-way ANOVA test with Tukey post-test was performed. All graph production and statistical analyses were done using GraphPad Prism software (La Jolla, CA).
Results and Discussion
B cells are not required for EAE induction or progression in PLP178–191-immunized B6 mice
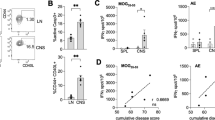
Prior studies have shown that B cells may play a protective role in MOG35–55-induced EAE14,20,21,31,32. However, it is not clear whether B cells play any role in other peptide-induced EAE B6 models and whether this phenomenon is generalizable. We therefore immunized WT B6 and B cell-deficient (μMT) mice with PLP178–191/CFA and monitored paralytic disease. We observed that B cell-deficient mice immunized with PLP178–191/CFA exhibited unaltered EAE progression, similar to that observed in WT mice (Fig. 1A). Cumulative disease index (CDI, or the summation of disease scores over time per mouse) was also not different (Fig. 1B). In contrast, MOG35–55/CFA-immunized B-cell deficient mice exhibited faster EAE kinetics and showed more severe disease (Fig. 1C,D), and is in accordance with previous literature20. This is also in contrast to observations in a susceptible BALB/c model, where B cells may play a regulatory role in the context of PLP180–199 or PLP185–206 peptide-induced EAE33. These data indicate that B cell engagement is suboptimal in PLP178–191-immunized B6 mice, and suggests that a short peptide-length PLP antigen is insufficient to drive either protective or pathogenic B cell responses in vivo in mice of this genetic background. These results also illustrate that it is not yet clear which PLP domains drive which B cell characteristics during disease.
B cells are not required for EAE induction or progression in PLP178–191-immunized B6 mice. WT or μMT mice were immunized s.c. with 100ug PLP178–191/CFA or MOG35–55/CFA on day 0 and given 250 ng pertussis toxin i.p. on days 0 and 2. (A,C) Clinical disease scores. (B,D) Cumulative disease index (CDI; measure of disease severity) depicts the summation of paralysis scores over time for each individual mouse in all groups. PLP178–191 groups: n = 30 WT; n = 30 μMT. MOG35–55 groups: n = 20 WT; n = 19 μMT. Data is representative of three independent experiments. ns = not significant; *p < 0.05; ****p < 0.0001.
Designed 83-mer peptide encompassing the extracellular domains of myelin proteolipid protein (PLPECD) elicits immune responses in vivo
It has been demonstrated that changes in MOG35–55/CFA-induced disease kinetics within μMT mice are due to the absence of naturally occurring IL-10-producing B1 Bregs14. This contrasts with switched high affinity follicular B cells, which may be pathogenic later in MOG35–55-induced disease14. Interestingly, in order to induce a B cell-dependent EAE model, one must immunize mice with full length recombinant hMOG protein rather than a smaller – albeit immunodominant – peptide (MOG35–55)13. Given the seemingly ancillary nature of B cells in PLP178–191-immunized mice (Fig. 1A,B), we hypothesized that a longer peptide (perhaps mimicking full length protein in a limited way) may provide more epitopes and structure with which B cells could potentially engage and process for presentation. We therefore designed a novel peptide encompassing both of two extracellular PLP domains, termed “PLPECD”. This is depicted in Fig. 2 in a diagram inspired by34 and24. PLP is an extremely hydrophobic multi-pass transmembrane protein (Fig. 2A). We therefore chose to incorporate extracellular sequences that excluded positions buried within, or just proximal to, the transmembrane region of the polypeptide structure while still containing as much of the immunodominant PLP178–191 epitope as possible (Fig. 2B). The final designed sequence was therefore an 83-mer spanning most of the two extracellular domains, including positions 179–191 from the immunodominant epitope (Fig. 2B).
Designed 83-mer peptide encompassing the extracellular domains of myelin proteolipid protein (PLPECD) elicits immune responses in vivo. (A) Multi-pass transmembrane orientation of PLP. Diagram is inspired by Hudson, 2004 (ref. 24) and Appikatla et al., 2014 (ref. 34). (B) Sequences corresponding to the extracellular domains of PLP were utilized to design a novel 83-mer polypeptide. WT B6 mice were immunized s.c. with either 100ug PLPECD/CFA or PLP178–191/CFA on day 0. (C,D) On day14, groups of PLPECD- and PLP178–191-immunized mice were challenged with PLP178–191 or PLPECD in the right and left ear pinnae of each mouse, respectively. Ears were measured at 48 h. (E,F) Reanalysis of data in (C,D) comparing similar ear challenges between two immunization groups (ie. white symbols in (C,D) are compared in (E) and black symbols in (C,D) are compared in (F). (G,H) Control experiments depicting 48 h ear swelling elicited by the indicated antigen challenges in mice immunized with PLP178–191 14 days prior. ns = not significant; *p < 0.05; ***p < 0.001.
Given that EAE is an immune-mediated model of demyelination35,36, we next determined the immunogenicity of PLPECD peptide by testing whether it could induce cellular immune responses in vivo. Type IV delayed-type hypersensitivity (DTH) assays have been utilized for decades as a measure of non-humoral, T cell-driven inflammation in vivo, and furthermore been used to read out immune responses to CNS myelin antigens30,37,38,39,40,41. Here, we performed DTH experiments as described previously30. Briefly, this involved making mice immune through s.c. flank injection with myelin peptide/CFA and subsequently challenging ear pinnae of immune mice with the same or similar myelin peptide (without CFA) or PBS alone (control) injection two weeks later. Using an engineer’s micrometer, measuring the ear thickness of injected ears at 48 h compared to ear thickness at a background 0 h measurement, one can identify “delta ear swelling” as a readout of the DTH reactions occurring in the challenged, immune animals. Specifically, individual mice were challenged with PLP178–191 in the right ear pinna and PLPECD in the left ear pinna 14 days following either PLP178–191- or PLPECD-immunization. Swelling was measured at 48 h post-ear challenge. PLPECD-immunized mice exhibited robust ear swelling when challenged with either PLPECD or PLP178–191 (Fig. 2C), indicating that PLPECD is sufficiently engaged, processed, and presented in these mice. As expected, PLP178–191-immunized mice exhibited robust swelling in PLP178–191-challenged ears (Fig. 2D, black symbols). PLPECD also induced DTH responses in PLP178–191-immunized mice (Fig. 2D, white symbols), albeit at lower levels than in PLPECD-immunized mice (Fig. 2E). This may be due to availability of epitopes on a per cell basis in vivo. PLP178–191-driven DTH was similarly elicited in both PLP178–191- and PLPECD-immune mice (Fig. 2F), indicating a robust induction of responses to this pathogenic epitope. Importantly, DTH does not develop when challenging ear pinnae of PLP178–191-immune mice with a control PBS alone injection (Fig. 2G) or with a non-cognate ovalbumin peptide (Fig. 2H). Collectively, these results demonstrate the immunogenicity of PLPECD.
PLPECD induces B cell-dependent EAE in B6 mice
To test whether PLPECD could successfully induce EAE, and to determine whether PLPECD could successfully engage B cells (either in a pathogenic or regulatory role), WT and B cell-deficient (µMT) B6 mice were actively immunized with PLPECD/CFA s.c. on day 0 along with i.p. injections of pertussis toxin on days 0 and 2. Clinical disease scores were monitored over time. Repeated experimentation composited in Fig. 3 demonstrates that EAE was indeed robustly induced in WT B6 mice following immunization with PLPECD (Fig. 3, black symbols). Intriguingly, µMT mice exhibited minimal disease induction (Fig. 3, white symbols). The two groups of mice were statistically different not only when comparing raw paralysis scores, but also with respect to day of onset, peak score of disease, and CDI (Fig. 3, EAE parameter table). These results ultimately suggest that PLPECD is a potent inducer of encephalitogenic responses in B6 mice, and that PLPECD-driven EAE disease is B cell-dependent.
PLPECD induces B cell-dependent EAE in B6 mice. WT or μMT mice were immunized s.c. with 100ug PLPECD/CFA on day 0 and given 250 ng pertussis toxin i.p. on days 0 and 2. Clinical disease scores were monitored and EAE parameters were analyzed (depicted in table as mean values ± standard error of the mean). Data presented includes scores from two to three independent experiments. ns = not significant; *p < 0.05; ***p < 0.001; ****p < 0.0001.
It is important to acknowledge that μMT mice could have compromised priming dynamics due to altered lymphoid and splenic architecture, resulting in priming loss brought about in a somewhat lymphocyte-extrinsic manner. However, this is not the case in our models, where despite the complications μMT mice present in these respects, Fig. 1 clearly shows equivalent EAE between μMT and WT mice when immunized with PLP178–191. Likewise, even in the face of B cell deficiency, PLP178–191-immune μMT and WT mice show similar PLP178–191-driven DTH reactions over background (Supplementary Fig. 1). This suggests that T cell priming in the context of PLP178–191 is not only largely unaffected, but is dominated by antigen presenting cell populations other than B cells (eg. dendritic cells). Priming is likely much different in the context of the larger antigen PLPECD. As demonstrated in Fig. 3, lack of B cells results in loss of robust EAE induction. Further, in the context of PLPECD immunization, lack of B cells results in a diminished PLP178–191-driven DTH reaction, similar to background levels (Supplementary Fig. 1). This result indicates that unlike PLP178–191 immunization, which is largely a B cell-independent priming, PLPECD requires B cells as a critical antigen-presenting cell for optimal T cell priming, and that other antigen presenting cells like dendritic cells are unable to fully liberate and present the bulk of the immunodominant epitope from the novel peptide. Future studies of PLPECD-induced germinal centers to establish how B cells interact with T cells in this model will be illuminating.
B cell-dependency in PLPECD-driven EAE disease in B6 mice occurs irrespective of antibody production
Functionally, the dependency of B cells in this model likely rests on antigen-presentation and/or pathogenic antibody production. It is known that oligoclonal switched IgG antibody bands within the CSF, along with B cells, are an indicator of MS disease9. Antibodies have also been shown to be pathogenic in some models of EAE. Antigen presentation function may also be important for B cell pathogenicity, as B cells have been shown to reactivate encephalitogenic CD4 T cells in the CNS of EAE mice15,16,17,18. Clearly, B cells play a role in MS as recent clinical trials have shown success with depleting B cells1,2,3. However, these depletion strategies target CD20+ B cells and leave behind antibody-producing plasma cells and plasma blasts. Given these data, in addition to those seen here, we wondered whether antigen presentation, rather than antibody production, explains the B cell-dependency of EAE induction in this model, perhaps reflecting their role in treated MS patients. To formally test this, we utilized AID−/−μS−/− mice42, which have a full complement of B cells, but lack the ability to secrete antibodies. We hypothesized that unlike μMT mice, which lack mature B cells and do not exhibit EAE disease, AID−/−μS−/− mice would be susceptible to EAE induction with PLPECD. Indeed, upon immunization, AID−/−μS−/− exhibited a similar disease course compared to WT mice (Fig. 4A). And as expected given the data in Fig. 3, μMT mice showed little EAE disease in comparison to their B cell-sufficient counterparts (Fig. 4A). In analyzing EAE disease parameters, AID−/−μS−/− mice were statistically indistinguishable from WT mice in raw paralysis scores exhibited over time, day of disease onset, peak disease score, and cumulative disease index compared to their μMT counterparts (Fig. 4A, table). These data indicate that the absence of antibody production by B cells was insufficient to render mice resistant to EAE induction. To confirm immune responses in these mice, DTH was elicited by challenging the right ear pinnae with PLP178–191 and the left ear pinnae with PBS alone (similar to that described in Fig. 2 above) on day 23 post-immunization. As expected, given the clinical score data in Fig. 4A and DTH data in Supplementary Fig. 1, μMT mice exhibited an inferior DTH reaction compared to their B cell-sufficient counterparts, indicating that the lack of antibodies had little effect in driving inflammatory responses and EAE (Fig. 4B), and further supports that antigen-presenting cells other than B cells (eg. dendritic cells) are insufficient for optimal T cell priming in PLPECD-immunized B6 mice.
B cell-dependency in PLPECD-driven EAE disease in B6 mice occurs irrespective of antibody production. (A) WT, μMT, and AID−/−μS−/− mice were immunized s.c. with 100ug PLPECD/CFA on day 0 and given 250 ng pertussis toxin i.p. on days 0 and 2. Clinical disease scores were monitored and EAE parameters were analyzed (depicted in table as mean values ± standard error of the mean). (B) On day 23 post-immunization, groups of mice in A were challenged with PLP178–191 or PBS alone in right and left ear pinnae, respectively. Ears were measured at 48 h. ns = not significant; *p < 0.05; ***p < 0.001; ****p < 0.0001.
Conclusions
We present here a novel B cell-dependent, PLP-driven murine EAE model. These results indicate that PLPECD engages WT B6 B cells more efficiently than a short, albeit immunodominant peptide (PLP178–191), leading to a likely pathogenic antigen-presenting cell role. This may ultimately mimic what is occurring in human MS patients, and warrants further study into whether depletion of PLPECD-driven B cells yields protection from demyelination. Any potential cellular and molecular immunological knowledge gained from this model may have increased translational implications given PLPECD utilizes sequences from the highly abundant (50% of total CNS myelin protein) and highly conserved (shares 100% amino acid sequence homology between mouse and human) myelin PLP. This work contributes an additional model to the field for investigating the pathogenesis and regulation of demyelinating disease, offering multiple ways for investigators to confirm immunological findings. PLPECD also provides an efficient way to investigate B cell-dependent demyelinating disease, as it obviates the need for various full-length protein expression systems in order to elude high titers of recombinant protein. It is also worth noting this study may represent a proof of principle that longer peptides (an 83-mer in this case) encompassing immunodominant sequences may be able to be designed for study of B cells’ role in other autoimmune diseases.
References
Hauser, S. L. et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. The New England journal of medicine 358, 676–688, https://doi.org/10.1056/NEJMoa0706383 (2008).
Hauser, S. L. et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. The New England journal of medicine 376, 221–234, https://doi.org/10.1056/NEJMoa1601277 (2017).
Montalban, X. et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. The New England journal of medicine 376, 209–220, https://doi.org/10.1056/NEJMoa1606468 (2017).
Mendel, I., Kerlero de Rosbo, N. & Ben-Nun, A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. European journal of immunology 25, 1951–1959, https://doi.org/10.1002/eji.1830250723 (1995).
Terry, R. L., Ifergan, I. & Miller, S. D. Experimental Autoimmune Encephalomyelitis in Mice. Methods in molecular biology 1304, 145–160, https://doi.org/10.1007/7651_2014_88 (2016).
Bettelli, E. et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. The Journal of experimental medicine 197, 1073–1081, https://doi.org/10.1084/jem.20021603 (2003).
Schluesener, H. J., Sobel, R. A., Linington, C. & Weiner, H. L. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. Journal of immunology 139, 4016–4021 (1987).
Cepok, S. et al. Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain 124, 2169–2176, https://doi.org/10.1093/brain/124.11.2169 (2001).
Weber, M. S., Hemmer, B. & Cepok, S. The role of antibodies in multiple sclerosis. Biochimica et biophysica acta 1812, 239–245, https://doi.org/10.1016/j.bbadis.2010.06.009 (2011).
Krumbholz, M., Derfuss, T., Hohlfeld, R. & Meinl, E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nature reviews. Neurology 8, 613–623, https://doi.org/10.1038/nrneurol.2012.203 (2012).
Lyons, J. A., Ramsbottom, M. J. & Cross, A. H. Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. European journal of immunology 32, 1905–1913, doi:10.1002/1521-4141(200207)32:7<1905::AID-IMMU1905>3.0.CO;2-L (2002).
Svensson, L. et al. A comparative analysis of B cell-mediated myelin oligodendrocyte glycoprotein-experimental autoimmune encephalomyelitis pathogenesis in B cell-deficient mice reveals an effect on demyelination. European journal of immunology 32, 1939–1946, doi:10.1002/1521-4141(200207)32:7<1939::AID-IMMU1939>3.0.CO;2-S (2002).
Lyons, J. A., San, M., Happ, M. P. & Cross, A. H. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. European journal of immunology 29, 3432–3439, doi:10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2 (1999).
Matsushita, T., Yanaba, K., Bouaziz, J. D., Fujimoto, M. & Tedder, T. F. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. The Journal of clinical investigation 118, 3420–3430, https://doi.org/10.1172/JCI36030 (2008).
Pierson, E. R., Stromnes, I. M. & Goverman, J. M. B cells promote induction of experimental autoimmune encephalomyelitis by facilitating reactivation of T cells in the central nervous system. Journal of immunology 192, 929–939, https://doi.org/10.4049/jimmunol.1302171 (2014).
Parker Harp, C. R. et al. B cell antigen presentation is sufficient to drive neuroinflammation in an animal model of multiple sclerosis. Journal of immunology 194, 5077–5084, https://doi.org/10.4049/jimmunol.1402236 (2015).
Parker Harp, C. R. et al. B cells are capable of independently eliciting rapid reactivation of encephalitogenic CD4 T cells in a murine model of multiple sclerosis. Plos one 13, e0199694, https://doi.org/10.1371/journal.pone.0199694 (2018).
Molnarfi, N. et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. The Journal of experimental medicine 210, 2921–2937, https://doi.org/10.1084/jem.20130699 (2013).
Wolf, S. D., Dittel, B. N., Hardardottir, F. & Janeway, C. A. Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. The Journal of experimental medicine 184, 2271–2278 (1996).
Yoshizaki, A. et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491, 264–268, https://doi.org/10.1038/nature11501 (2012).
Fillatreau, S., Sweenie, C. H., McGeachy, M. J., Gray, D. & Anderton, S. M. B cells regulate autoimmunity by provision of IL-10. Nature immunology 3, 944–950, https://doi.org/10.1038/ni833 (2002).
Marta, C. B., Oliver, A. R., Sweet, R. A., Pfeiffer, S. E. & Ruddle, N. H. Pathogenic myelin oligodendrocyte glycoprotein antibodies recognize glycosylated epitopes and perturb oligodendrocyte physiology. Proceedings of the National Academy of Sciences of the United States of America 102, 13992–13997, https://doi.org/10.1073/pnas.0504979102 (2005).
Oliver, A. R., Lyon, G. M. & Ruddle, N. H. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. Journal of immunology 171, 462–468 (2003).
Hudson, L. D. In Myelin Biology and Disorders Vol. 1 Ch. 16, 401–420 (Elsevier Academic Press, 2004).
Tuohy, V. K., Lu, Z., Sobel, R. A., Laursen, R. A. & Lees, M. B. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. Journal of immunology 142, 1523–1527 (1989).
Greer, J. M., Kuchroo, V. K., Sobel, R. A. & Lees, M. B. Identification and characterization of a second encephalitogenic determinant of myelin proteolipid protein (residues 178–191) for SJL mice. Journal of immunology 149, 783–788 (1992).
Ortega, S. B., Kashi, V. P., Cunnusamy, K., Franco, J. & Karandikar, N. J. Autoregulatory CD8 T cells depend on cognate antigen recognition and CD4/CD8 myelin determinants. Neurology(R) neuroimmunology & neuroinflammation 2, e170, https://doi.org/10.1212/NXI.0000000000000170 (2015).
Kashi, V. P., Ortega, S. B. & Karandikar, N. J. Neuroantigen-specific autoregulatory CD8+ T cells inhibit autoimmune demyelination through modulation of dendritic cell func tion. Plos one 9, e105763, https://doi.org/10.1371/journal.pone.0105763 (2014).
Itani, F. R. et al. Suppression of autoimmune demyelinating disease by preferential stimulation of CNS-specific CD8 T cells using Listeria-encoded neuroantigen. Scientific reports 7, 1519, https://doi.org/10.1038/s41598-017-01771-8 (2017).
Boyden, A. W., Brate, A. A. & Karandikar, N. J. Early IFNgamma-Mediated and Late Perforin-Mediated Suppression of Pathogenic CD4 T Cell Responses Are Both Required for Inhibition of Demyelinating Disease by CNS-Specific Autoregulatory CD8 T Cells. Front Immunol 9, 2336, https://doi.org/10.3389/fimmu.2018.02336 (2018).
Matsushita, T., Horikawa, M., Iwata, Y. & Tedder, T. F. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. Journal of immunology 185, 2240–2252, https://doi.org/10.4049/jimmunol.1001307 (2010).
Ray, A., Mann, M. K., Basu, S. & Dittel, B. N. A case for regulatory B cells in controlling the severity of autoimmune-mediated inflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. Journal of neuroimmunology 230, 1–9, https://doi.org/10.1016/j.jneuroim.2010.10.037 (2011).
Lyons, J. A., Ramsbottom, M. J., Mikesell, R. J. & Cross, A. H. B cells limit epitope spreading and reduce severity of EAE induced with PLP peptide in BALB/c mice. Journal of autoimmunity 31, 149–155, https://doi.org/10.1016/j.jaut.2008.04.025 (2008).
Appikatla, S. et al. Insertion of proteolipid protein into oligodendrocyte mitochondria regulates extracellular pH and adenosine triphosphate. Glia 62, 356–373, https://doi.org/10.1002/glia.22591 (2014).
Ben-Nun, A., Wekerle, H. & Cohen, I. R. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. European journal of immunology 11, 195–199, https://doi.org/10.1002/eji.1830110307 (1981).
Stromnes, I. M. & Goverman, J. M. Passive induction of experimental allergic encephalomyelitis. Nature protocols 1, 1952–1960, https://doi.org/10.1038/nprot.2006.284 (2006).
Tompkins, S. M. et al. De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis. Journal of immunology 168, 4173–4183 (2002).
Croxford, J. L., Olson, J. K., Anger, H. A. & Miller, S. D. Initiation and exacerbation of autoimmune demyelination of the central nervous system via virus-induced molecular mimicry: implications for the pathogenesis of multiple sclerosis. Journal of virology 79, 8581–8590, https://doi.org/10.1128/JVI.79.13.8581-8590.2005 (2005).
Smith, C. E. & Miller, S. D. Multi-peptide coupled-cell tolerance ameliorates ongoing relapsing EAE associated with multiple pathogenic autoreactivities. Journal of autoimmunity 27, 218–231, https://doi.org/10.1016/j.jaut.2006.12.002 (2006).
Farooq, S. M. & Ashour, H. M. Eye-mediated induction of specific immune tolerance to encephalitogenic antigens. CNS neuroscience & therapeutics 19, 503–510, https://doi.org/10.1111/cns.12087 (2013).
Farooq, S. M. & Ashour, H. M. In vitro-induced cell-mediated immune deviation to encephalitogenic antigens. Brain, behavior, and immunity 35, 64–69, https://doi.org/10.1016/j.bbi.2013.09.016 (2014).
Kumazaki, K. et al. AID−/−mus−/− mice are agammaglobulinemic and fail to maintain B220-CD138+ plasma cells. Journal of immunology 178, 2192–2203, https://doi.org/10.4049/jimmunol.178.4.2192 (2007).
Acknowledgements
The authors thank Drs. Sushmita Sinha, Ashutosh Mangalam, Scott Lieberman, Ali Jabbari and Noah Butler at the University of Iowa for critical evaluation and discussion. The authors thank Drs. Frances Lund and Troy Randall at the University of Alabama at Birmingham and Dr. Tasuku Honjo at Kyoto University, Japan for providing the AID−/−μS−/− mice. The authors acknowledge funding support in part to NJK from the National Institutes of Health (R01AI09106) and the US Department of Veterans Affairs (I01BX003677), and to AWB from the National Multiple Sclerosis Society (Fellowship Award FG 2094-A-1).
Author information
Authors and Affiliations
Contributions
A.W.B. and N.J.K. conceived and designed the study. A.W.B. and A.A.B. performed the experiments. A.W.B. organized the data and wrote the manuscript. A.W.B., A.A.B. and N.J.K. contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boyden, A.W., Brate, A.A. & Karandikar, N.J. Novel B cell-dependent multiple sclerosis model using extracellular domains of myelin proteolipid protein. Sci Rep 10, 5011 (2020). https://doi.org/10.1038/s41598-020-61928-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61928-w
This article is cited by
-
SLAMF7 modulates B cells and adaptive immunity to regulate susceptibility to CNS autoimmunity
Journal of Neuroinflammation (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.