Abstract
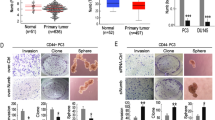
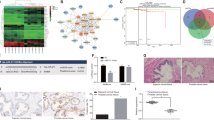
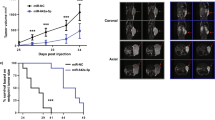
Cancer stem cells (CSCs), or tumor-initiating cells, are involved in tumor progression and metastasis1. MicroRNAs (miRNAs) regulate both normal stem cells and CSCs2,3,4,5, and dysregulation of miRNAs has been implicated in tumorigenesis6. CSCs in many tumors—including cancers of the breast7, pancreas8, head and neck9, colon10,11, small intestine12, liver13, stomach14, bladder15 and ovary16—have been identified using the adhesion molecule CD44, either individually or in combination with other marker(s). Prostate CSCs with enhanced clonogenic17 and tumor-initiating and metastatic18,19 capacities are enriched in the CD44+ cell population, but whether miRNAs regulate CD44+ prostate cancer cells and prostate cancer metastasis remains unclear. Here we show, through expression analysis, that miR-34a, a p53 target20,21,22,23,24, was underexpressed in CD44+ prostate cancer cells purified from xenograft and primary tumors. Enforced expression of miR-34a in bulk or purified CD44+ prostate cancer cells inhibited clonogenic expansion, tumor regeneration, and metastasis. In contrast, expression of miR-34a antagomirs in CD44− prostate cancer cells promoted tumor development and metastasis. Systemically delivered miR-34a inhibited prostate cancer metastasis and extended survival of tumor-bearing mice. We identified and validated CD44 as a direct and functional target of miR-34a and found that CD44 knockdown phenocopied miR-34a overexpression in inhibiting prostate cancer regeneration and metastasis. Our study shows that miR-34a is a key negative regulator of CD44+ prostate cancer cells and establishes a strong rationale for developing miR-34a as a novel therapeutic agent against prostate CSCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Visvader, J.E. & Lindeman, G.J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768 (2008).
Croce, C.M. & Calin, G.A. miRNAs, cancer, and stem cell division. Cell 122, 6–7 (2005).
Melton, C., Judson, R.L. & Blelloch, R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463, 621–626 (2010).
Yu, F. et al. let-7 regulates self-renewal and tumorigenicity of breast cancer cells. Cell 131, 1109–1123 (2007).
Shimono, Y. et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 138, 592–603 (2009).
Esquela-Kerscher, A. & Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 6, 259–269 (2006).
Al-Hajj, M., Wicha, M.S., Benito-Hernandez, A., Morrison, S.J. & Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 100, 3983–3988 (2003).
Li, C. et al. Identification of pancreatic cancer stem cells. Cancer Res. 67, 1030–1037 (2007).
Prince, M.E. et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 104, 973–978 (2007).
Dalerba, P. et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. USA 104, 10158–10163 (2007).
Du, L. et al. CD44 is of functional importance for colorectal cancer stem cells. Clin. Cancer Res. 14, 6751–6760 (2008).
Zeilstra, J. et al. Deletion of the WNT target and cancer stem cell marker CD44 in Apc(Min/+) mice attenuates intestinal tumorigenesis. Cancer Res. 68, 3655–3661 (2008).
Yang, Z.F. et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 13, 153–166 (2008).
Takaishi, S. et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 27, 1006–1020 (2009).
Chan, K.S. et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. USA 106, 14016–14021 (2009).
Zhang, S. et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 68, 4311–4320 (2008).
Collins, A.T., Berry, P.A., Hyde, C., Stower, M.J. & Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65, 10946–10951 (2005).
Patrawala, L. et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 25, 1696–1708 (2006).
Patrawala, L., Calhoun-Davis, T., Schneider-Broussard, R. & Tang, D.G. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+α2β1+ cell population is enriched in tumor-initiating cells. Cancer Res. 67, 6796–6805 (2007).
He, L. et al. A microRNA component of the p53 tumour suppressor network. Nature 447, 1130–1134 (2007).
Raver-Shapira, N. et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell 26, 731–743 (2007).
Chang, T.C. et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell 26, 745–752 (2007).
Bommer, G.T. et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr. Biol. 17, 1298–1307 (2007).
Tarasov, V. et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle 6, 1586–1593 (2007).
Johnson, C.D. et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 67, 7713–7722 (2007).
Wiggins, J.F. et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 70, 5923–5930 (2010).
Jeter, C.R. et al. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells 27, 993–1005 (2009).
Patrawala, L. et al. Side population (SP) is enriched in tumorigenic, stem-like cancer cells whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 65, 6207–6219 (2005).
Bhatia, B. et al. Critical and distinct roles of p16 and telomerase in regulating the proliferative lifespan of normal human prostate epithelial progenitor cells. J. Biol. Chem. 283, 27957–27972 (2008).
Li, H.W. et al. Methodologies in assaying prostate cancer stem cells. Methods Mol. Biol. 568, 85–138 (2009).
Hermeking, H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 17, 193–199 (2010).
Li, H., Chen, X., Calhoun-Davis, T., Claypool, K. & Tang, D.G. PC3 Human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res. 68, 1820–1825 (2008).
Dontu, G. et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17, 1253–1270 (2003).
Yamakuchi, M., Ferlito, M. & Lowenstein, C.J. miR-34a repression of SIRT1 regulates apoptosis. Proc. Natl. Acad. Sci. USA 105, 13421–13426 (2008).
Li, Y. et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 69, 7569–7576 (2009).
Miranda, K.C. et al. A pattern-based method for the identification of microRNA-target sites and their corresponding RNA/RNA complexes. Cell 126, 1203–1217 (2006).
Godar, S. et al. Growth-inhibitory and tumor-suppressive functions of p53 depend on its repression of CD44 expression. Cell 134, 62–73 (2008).
Jin, L., Hope, K.J., Zhai, Q., Smadja-Joffe, F. & Dick, J.E. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med. 12, 1167–1174 (2006).
Ji, Q. et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE 4, e6816 (2009).
Acknowledgements
We thank K. Claypool and P. Whitney for FACS, the Histology Core for help with immunohistochemistry, K. Lin for statistical analysis, G. Calin for critically reading the manuscript and other members of the Tang lab for support and discussions. We also thank G. Hannon (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, USA) for the MSCV-PIG vector. This work was supported in part by grants from the US National Institutes of Health (R01-AG023374, R01-ES015888, R21-ES015893, R21-CA150009), the US Department of Defense (W81XWH-07-1-0616, W81XWH-08-1-0472) and Elsa Pardee Foundation (D.G.T.) and by two M.D. Anderson Cancer Center grants (CCSG-5 P30 CA016672-34 and ES007784). C. Liu and H. Li were supported in part by predoctoral fellowships from the US Department of Defense.
Author information
Authors and Affiliations
Contributions
C.L., K.K., B.L., X.C. and L.P. designed and performed the experiments with help from C.J., T.C.-D., H.L., S.H., H.Y., J.F.W. and A.G.B., R.F. provided all HPCa samples. C.L. and D.G.T. prepared the manuscript. D.G.T., with help from D.B., designed the experiments and supervised the whole project. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
K.K, J.F.W, A.G.B. and D.B are employees of Mirna Therapeutics, Inc., which develops miRNA-based therapeutics.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Methods, Supplementary Figures 1–15 and Supplementary Tables 1 and 2 (PDF 2176 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Kelnar, K., Liu, B. et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 17, 211–215 (2011). https://doi.org/10.1038/nm.2284
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2284
This article is cited by
-
Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer
Molecular Cancer (2023)
-
Targeted therapy using nanocomposite delivery systems in cancer treatment: highlighting miR34a regulation for clinical applications
Cancer Cell International (2023)
-
A first-in-class fully modified version of miR-34a with outstanding stability, activity, and anti-tumor efficacy
Oncogene (2023)
-
p53 downregulates PD-L1 expression via miR-34a to inhibit the growth of triple-negative breast cancer cells: a potential clinical immunotherapeutic target
Molecular Biology Reports (2023)
-
Extirpating the cancer stem cell hydra: Differentiation therapy and Hyperthermia therapy for targeting the cancer stem cell hierarchy
Clinical and Experimental Medicine (2023)