Abstract
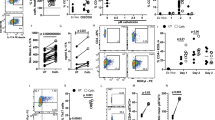
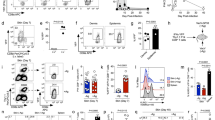
Leukotriene B4 (LTB4) is a potent chemoattractant for myeloid leukocytes, which express BLT1, the high-affinity receptor for LTB4. We report here that BLT1 is induced substantially in CD8+ effector T cells and at lower amounts in CD8+ central memory T cells. LTB4 elicited BLT1-dependent chemotaxis in effector cells, but not in naive or central memory cells. Intravital microscopy showed that BLT1 signaling induced rapid integrin-mediated arrest of rolling effector and central memory cells in postcapillary venules. In competitive homing experiments, wild-type effector cells were three times more efficient at migrating to the inflamed peritoneal cavity than were BLT-deficient effector cells. These results identify LTB4-BLT1 as a potent nonchemokine pathway for cytotoxic effector cell traffic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
von Andrian, U.H. & Mackay, C.R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343, 1020–1034 (2000).
Banchereau, J. et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 (2000).
Kaech, S.M. & Ahmed, R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2, 415–422 (2001).
Xie, H., Lim, Y.C., Luscinskas, F.W. & Lichtman, A.H. Acquisition of selectin binding and peripheral homing properties by CD4+ and CD8+ T cells. J. Exp. Med. 189, 1765–1776 (1999).
Weninger, W., Crowley, M.A., Manjunath, N. & von Andrian, U.H. Migratory properties of naive, effector, and memory CD8+ T cells. J. Exp. Med. 194, 953–966 (2001).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Manjunath, N. et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 108, 871–878 (2001).
Lauvau, G. et al. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science 294, 1735–1739 (2001).
Wherry, E.J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4, 225–234 (2003).
Weninger, W., Manjunath, N. & von Andrian, U.H. Migration and differentiation of CD8+ T cells. Immunol. Rev. 185, 221–233 (2002).
Campbell, J.J. & Butcher, E.C. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 12, 336–341 (2000).
Samuelsson, B., Dahlen, S.E., Lindgren, J.A., Rouzer, C.A. & Serhan, C.N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science 237, 1171–1176 (1987).
Lewis, R.A., Austen, K.F. & Soberman, R.J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N. Engl. J. Med. 323, 645–655 (1990).
Ford-Hutchinson, A.W., Bray, M.A., Doig, M.V., Shipley, M.E. & Smith, M.J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 286, 264–265 (1980).
Serhan, C.N. & Prescott, S.M. The scent of a phagocyte: Advances on leukotriene B4 receptors. J. Exp. Med. 192, F5–F8 (2000).
Kato, K., Yokomizo, T., Izumi, T. & Shimizu, T. Cell-specific transcriptional regulation of human leukotriene B4 receptor gene. J. Exp. Med. 192, 413–420 (2000).
Yokomizo, T., Kato, K., Terawaki, K., Izumi, T. & Shimizu, T. A second leukotriene B4 receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 192, 421–432 (2000).
Tager, A.M. et al. BLTR mediates leukotriene B4-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J. Exp. Med. 192, 439–446 (2000).
Haribabu, B. et al. Targeted disruption of the leukotriene B4 receptor in mice reveals its role in inflammation and platelet-activating factor-induced anaphylaxis. J. Exp. Med. 192, 433–438 (2000).
Payan, D.G., Missirian-Bastian, A. & Goetzl, E.J. Human T-lymphocyte subset specificity of the regulatory effects of leukotriene B4 . Proc. Natl. Acad. Sci. USA 81, 3501–3505 (1984).
Goetzl, E.J. et al. Receptor-specific mechanisms for the responses of human leukocytes to leukotrienes. Ann. NY Acad. Sci. 524, 345–355 (1988).
Showell, H.J., Breslow, R., Conklyn, M.J., Hingorani, G.P. & Koch, K. Characterization of the pharmacological profile of the potent LTB4 antagonist CP-105,696 on murine LTB4 receptors in vitro. Br. J. Pharmacol. 117, 1127–1132 (1996).
Weninger, W. et al. Naive T cell recruitment to non-lymphoid tissues: a role for endothelium-expressed CCL21 in autoimmune disease and lymphoid neogenesis. J. Immunol. 170, 4638–4648 (2003).
Coxon, A. et al. A novel role for the β2 integrin CD11b/CD18 in neutrophil apoptosis: A homeostatic mechanism in inflammation. Immunity 5, 653–666 (1996).
Rosengren, S., Olofsson, A.M., von Andrian, U.H., Lundgren-Akerlund, E. & Arfors, K.-E. Leukotriene B4-induced neutrophil-mediated endothelial leakage in vitro and in vivo. J. Appl. Physiol. 71, 1322–1330 (1991).
von Andrian, U.H. et al. L-selectin function is required for β2-integrin-mediated neutrophil adhesion at physiological shear rates in vivo. Am. J. Physiol. 263, H1034–H1044 (1992).
Butcher, E.C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 67, 1033–1036 (1991).
Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multi-step paradigm. Cell 76, 301–314 (1994).
Ley, K. et al. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 181, 669–675 (1995).
Ley, K., Allietta, M., Bullard, D.C. & Morgan, S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ. Res. 83, 287–294 (1998).
Nohgawa, M. et al. Leukotriene B4-activated human endothelial cells promote transendothelial neutrophil migration. J. Leukoc. Biol. 62, 203–209 (1997).
Funk, C.D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 (2001).
Yokomizo, T., Izumi, T. & Shimizu, T. Leukotriene B4: metabolism and signal transduction. Arch. Biochem. Biophys. 385, 231–241 (2001).
Campbell, D.J. & Butcher, E.C. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195, 135–141 (2002).
Turner, C.R. et al. In vitro and in vivo effects of leukotriene B4 antagonism in a primate model of asthma. J. Clin. Invest. 97, 381–387 (1996).
Gladue, R.P. et al. Inhibition of leukotriene B4-receptor interaction suppresses eosinophil infiltration and disease pathology in a murine model of experimental allergic encephalomyelitis. J. Exp. Med. 183, 1893–1898 (1996).
Griffiths, R.J. et al. Collagen-induced arthritis is reduced in 5-lipoxygenase-activating protein-deficient mice. J. Exp. Med. 185, 1123–1129 (1997).
Aiello, R.J. et al. Leukotriene B4 receptor antagonism reduces monocytic foam cells in mice. Arterioscler. Thromb. Vasc. Biol. 22, 443–449 (2002).
Matsukawa, A. et al. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J. Immunol. 163, 6148–6154 (1999).
Foxman, E.F., Kunkel, E.J. & Butcher, E.C. Integrating conflicting chemotactic signals. The role of memory in leukocyte navigation. J. Cell Biol. 147, 577–588 (1999).
Campbell, J.J., Qin, S., Bacon, K.B., Mackay, C.R. & Butcher, E.C. Biology of chemokine and classical chemoattractant receptors: Differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J. Cell Biol. 134, 255–266 (1996).
Huang, W.W. et al. Molecular and biological characterization of the murine leukotriene B4 receptor expressed on eosinophils. J. Exp. Med. 188, 1063–1074 (1998).
Acknowledgements
We thank C. Schweitzer, B. Reinhardt and G. Cheng for technical support, and J. Moore for editorial assistance. We also thank W. Weninger and I. Mazo for advice and discussions. This work was supported by a National Institutes of Health T32 Transfusion Biology and Medicine Training Grant from Children's Hospital, Boston (to K.G. and M.G.) and by National Institutes of Health grants HL48675, HL54936 and HL56949 to U.H.v.A; HL04087 to A.M.T.; and AI050892, AI46999 and DK5005 to A.D.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Goodarzi, K., Goodarzi, M., Tager, A. et al. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol 4, 965–973 (2003). https://doi.org/10.1038/ni972
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni972
This article is cited by
-
Activation of leukotriene B4 receptor 1 is a prerequisite for complement receptor 3-mediated antifungal responses of neutrophils
Cellular & Molecular Immunology (2024)
-
A review of non-prostanoid, eicosanoid receptors: expression, characterization, regulation, and mechanism of action
Journal of Cell Communication and Signaling (2022)
-
Expression of leukotriene B4 receptor 1 defines functionally distinct DCs that control allergic skin inflammation
Cellular & Molecular Immunology (2021)
-
Baicalin and Geniposide Inhibit Polarization and Inflammatory Injury of OGD/R-Treated Microglia by Suppressing the 5-LOX/LTB4 Pathway
Neurochemical Research (2021)
-
Early and Sustained Increases in Leukotriene B4 Levels Are Associated with Poor Clinical Outcome in Ischemic Stroke Patients
Neurotherapeutics (2020)