Abstract
Caveolin-1 (Cav1) regulates the nanoscale organization and compartmentalization of the plasma membrane. Here we found that Cav1 controlled the distribution of nanoclusters of isotype-specific B cell antigen receptors (BCRs) on the surface of B cells. In mature B cells stimulated with antigen, the immunoglobulin M BCR (IgM-BCR) gained access to lipid domains enriched for GM1 glycolipids, by a process that was dependent on the phosphorylation of Cav1 by the Src family of kinases. Antigen-induced reorganization of nanoclusters of IgM-BCRs and IgD-BCRs regulated BCR signaling in vivo. In immature Cav1-deficient B cells, altered nanoscale organization of IgM-BCRs resulted in a failure of receptor editing and a skewed repertoire of B cells expressing immunoglobulin-μ heavy chains with hallmarks of poly- and auto-reactivity, which ultimately led to autoimmunity in mice. Thus, Cav1 emerges as a cell-intrinsic regulator that prevents B cell–induced autoimmunity by means of its role in plasma-membrane organization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hombach, J., Tsubata, T., Leclercq, L., Stappert, H. & Reth, M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature 343, 760–762 (1990).
Reth, M. Antigen receptor tail clue. Nature 338, 383–384 (1989).
Kurosaki, T. Genetic analysis of B cell antigen receptor signaling. Annu. Rev. Immunol. 17, 555–592 (1999).
Rolli, V. et al. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol. Cell 10, 1057–1069 (2002).
Meffre, E., Casellas, R. & Nussenzweig, M.C. Antibody regulation of B cell development. Nat. Immunol. 1, 379–385 (2000).
Tonegawa, S. Somatic generation of antibody diversity. Nature 302, 575–581 (1983).
Bassing, C.H., Swat, W. & Alt, F.W. The mechanism and regulation of chromosomal V(D)J recombination. Cell 109 (Suppl.), S45–S55 (2002).
Gu, H., Tarlinton, D., Müller, W., Rajewsky, K. & Förster, I. Most peripheral B cells in mice are ligand selected. J. Exp. Med. 173, 1357–1371 (1991).
Shlomchik, M.J. Sites and stages of autoreactive B cell activation and regulation. Immunity 28, 18–28 (2008).
Retter, M.W. & Nemazee, D. Receptor editing occurs frequently during normal B cell development. J. Exp. Med. 188, 1231–1238 (1998).
Casellas, R. et al. Contribution of receptor editing to the antibody repertoire. Science 291, 1541–1544 (2001).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Menard, L. et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J. Clin. Invest. 121, 3635–3644 (2011).
Ng, Y.S., Wardemann, H., Chelnis, J., Cunningham-Rundles, C. & Meffre, E. Bruton's tyrosine kinase is essential for human B cell tolerance. J. Exp. Med. 200, 927–934 (2004).
Grimaldi, C.M., Hicks, R. & Diamond, B. B cell selection and susceptibility to autoimmunity. J. Immunol. 174, 1775–1781 (2005).
Schamel, W.W.A. & Reth, M. Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity 13, 5–14 (2000).
Yang, J. & Reth, M. Oligomeric organization of the B-cell antigen receptor on resting cells. Nature 467, 465–469 (2010).
Kläsener, K., Maity, P.C., Hobeika, E., Yang, J. & Reth, M. B cell activation involves nanoscale receptor reorganizations and inside-out signaling by Syk. eLife 3, e02069 (2014).
Maity, P.C., Yang, J., Klaesener, K. & Reth, M. The nanoscale organization of the B lymphocyte membrane. BBA - Mol. Cell Res. 1853, 830–840 (2015).
Mattila, P.K. et al. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity 38, 461–474 (2013).
Parton, R.G. & del Pozo, M.A. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 14, 98–112 (2013).
Goetz, J.G. et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146, 148–163 (2011).
Gaus, K., Le Lay, S., Balasubramanian, N. & Schwartz, M.A. Integrin-mediated adhesion regulates membrane order. J. Cell Biol. 174, 725–734 (2006).
Hernández-Deviez, D.J. et al. Caveolin regulates endocytosis of the muscle repair protein, dysferlin. J. Biol. Chem. 283, 6476–6488 (2008).
Hoffmann, C. et al. Caveolin limits membrane microdomain mobility and integrin-mediated uptake of fibronectin-binding pathogens. J. Cell Sci. 123, 4280–4291 (2010).
Fra, A.M., Williamson, E., Simons, K. & Parton, R.G. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J. Biol. Chem. 269, 30745–30748 (1994).
Tomassian, T. et al. Caveolin-1 orchestrates TCR synaptic polarity, signal specificity, and function in CD8 T cells. J. Immunol. 187, 2993–3002 (2011).
Schönle, A. et al. Caveolin-1 regulates TCR signal strength and regulatory T-cell differentiation into alloreactive T cells. Blood 127, 1930–1939 (2016).
Medina, F.A., Williams, T.M., Sotgia, F., Tanowitz, H.B. & Lisanti, M.P. A novel role for caveolin-1 in B lymphocyte function and the development of thymus-independent immune responses. Cell Cycle 5, 1865–1871 (2006).
Drab, M. et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293, 2449–2452 (2001).
Kenworthy, A.K., Petranova, N. & Edidin, M. High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol. Biol. Cell 11, 1645–1655 (2000).
Schmitz, R., Baumann, G. & Gram, H. Catalytic specificity of phosphotyrosine kinases Blk, Lyn, c-Src and Syk as assessed by phage display. J. Mol. Biol. 260, 664–677 (1996).
Cao, H., Courchesne, W.E. & Mastick, C.C. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J. Biol. Chem. 277, 8771–8774 (2002).
Maity, P.C. et al. B cell antigen receptors of the IgM and IgD classes are clustered in different protein islands that are altered during B cell activation. Sci. Signal. 8, ra93 (2015).
Lee, W.-Y. & Tolar, P. Activation of the B cell receptor leads to increased membrane proximity of the Igα cytoplasmic domain. PLoS One 8, e79148 (2013).
Razani, B. et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 276, 38121–38138 (2001).
Park, D.S. et al. Caveolin-1 null (−/−) mice show dramatic reductions in life span. Biochemistry 42, 15124–15131 (2003).
Gay, D., Saunders, T., Camper, S. & Weigert, M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 177, 999–1008 (1993).
Pelanda, R. et al. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity 7, 765–775 (1997).
Treanor, B., Depoil, D., Bruckbauer, A. & Batista, F.D. Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J. Exp. Med. 208, 1055–1068 (2011).
Vargas, L. et al. Functional interaction of caveolin-1 with Bruton's tyrosine kinase and Bmx. J. Biol. Chem. 277, 9351–9357 (2002).
Jo, A. et al. SHP-2 binds to caveolin-1 and regulates Src activity via competitive inhibition of CSK in response to H2O2 in astrocytes. PLoS One 9, e91582 (2014).
Radel, C. & Rizzo, V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am. J. Physiol. Heart Circ. Physiol. 288, H936–H945 (2005).
Gupta, N. & DeFranco, A.L. Lipid rafts and B cell signaling. Semin. Cell Dev. Biol. 18, 616–626 (2007).
Muriel, O. et al. Phosphorylated filamin A regulates actin-linked caveolae dynamics. J. Cell Sci. 124, 2763–2776 (2011).
Sohn, H.W., Tolar, P. & Pierce, S.K. Membrane heterogeneities in the formation of B cell receptor-Lyn kinase microclusters and the immune synapse. J. Cell Biol. 182, 367–379 (2008).
Huck, S., Le Corre, R., Youinou, P. & Zouali, M. Expression of B cell receptor-associated signaling molecules in human lupus. Autoimmunity 33, 213–224 (2001).
Sproul, T.W., Malapati, S., Kim, J. & Pierce, S.K. Cutting edge: B cell antigen receptor signaling occurs outside lipid rafts in immature B cells. J. Immunol. 165, 6020–6023 (2000).
Kim, K.J., Kanellopoulos-Langevin, C., Merwin, R.M., Sachs, D.H. & Asofsky, R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 122, 549–554 (1979).
Hobeika, E. et al. Testing gene function early in the B cell lineage in mb1-cre mice. Proc. Natl. Acad. Sci. USA 103, 13789–13794 (2006).
Navarro-Lérida, I. et al. A palmitoylation switch mechanism regulates Rac1 function and membrane organization. EMBO J. 31, 534–551 (2012).
Seppälä, I.J. & Mäkelä, O. Adjuvant effect of bacterial LPS and/or alum precipitation in responses to polysaccharide and protein antigens. Immunology 53, 827–836 (1984).
Fiala, G.J. et al. Kidins220/ARMS binds to the B cell antigen receptor and regulates B cell development and activation. J. Exp. Med. 212, 1693–1708 (2015).
Belver, L., de Yébenes, V.G. & Ramiro, A.R. MicroRNAs prevent the generation of autoreactive antibodies. Immunity 33, 713–722 (2010).
Acknowledgements
We thank the BIOSS toolbox for reagents; M.C. Guadamillas and A. Cerezo for the generation of the B6.Cav1Y14F/Y14F mice; E. Hobeika (Max Planck Institute of Immunology and Epigenetics) for Cd79a−/− mice; H. Jumaa (Max Planck Institute of Immunology and Epigenetics) for 3-83Igi mice; C. Johner, U. Stauffer, N. Joswig, and K. Fehrenbach for experimental help; Y. Kulathu, M. Swamy M. Rizzi, K. Schachtrup and Y.R. Carrasco for critical reading of the manuscript; and W. Schamel for intellectual input and scientific discussions. Supported by the German Research Foundation (DFG) (SFB1160 IMPATH P5 to S.M., supporting F.A.H. and A.-M.S.; MI1942/2-1 to S.M., supporting G.J.F.; the Spemann Graduate School (Excellence Initiative GSC-4 to K.R.); the BIOSS Centre for Biological Signalling Studies (EXC294 to S.M. and M.R.); TRR130-P02 to M.R.; and SFB746-P07 to M.R), the European Research Council (32297 to M.R.), the Spanish Ministry of Economy and Competitiveness (SAF2008-02100 (support for S.M. in 2008); SAF2011-25047, CSD2009-00016 and SAF2014-51876-R to M.A.D.P.; and support for CNIC), the Worldwide Cancer Research Foundation (15-0404 to M.A.D.P.), the Ramón y Cajal Program from the MINECO (2009-2011 to S.M.), Asociación Española Contra el Cáncer (I.N.-L.), the Pro-CNIC Foundation (support for CNIC) and Severo Ochoa Center of Excellence (SEV-2015-0505 for CNIC).
Author information
Authors and Affiliations
Contributions
S.M. designed this study, performed experiments and wrote the manuscript with input from all authors; K.K. and A.-M.S. performed the PLA experiments; G.J.F. and F.A.H. performed ex vivo stimulation; T.O.-I. raised the mice and performed experiments; K.R. and M.S. performed kidney analysis; I.N.-L. performed DRM experiments; M.R. provided intellectual input and conceptual and scientific advice; M.A.D.P. provided B6.Cav1Y14F/Y14F mice, Cav1 expertise to the conception of the project and intellectual input and conceptual and scientific advice; and all authors critically read the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
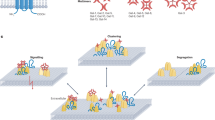
Supplementary Figure 1 Cav1 regulates the proximity of the IgM-BCR to GM1 on the surface of B cells.
(a) Purified B and T cells from the spleen of the indicated mice were lysed. As a comparative control, lung tissue was homogenized and lysed. Proteins were then separated by SDS-PAGE, Cav1 and tubulin were detected by immunoblot. The total amount of protein loaded is indicated. A representative experiment out of 2 independently performed experiments is shown. (b) Schematics of the IgM:CTxB PLA experimental setup. Analysis of the proximity of the IgM-BCR to GM1 was performed using an oligo-labeled anti-IgM Fab (grey) and direct oligo-labeled GM1-bound CTxB (purple). PLA signal is indicated with a red star. (c) PLA controls show single anti-IgM Fab or CTxB on unstimulated (top) and 5 min 0.5 mM pervanadate (perV) stimulated (bottom) B6.WT splenic B cells. The PLA was performed as described in (b). Representative microscope images show DAPI stained nuclei (in blue) and PLA signals (in red). A representative experiment out of 7 is shown. (d) Purified splenic B cells were stimulated with 10 μg/ml of anti-IgM (Fab‘)2 for the indicated times or left unstimulated. Cells were fixed and in situ IgM:CTxB PLA performed. PLA signals were counted and normalized to the PLA signals of WT cells stimulated for 5 min with perV for each individual experiment. (e) K46μml cells were lentivirally transfected to express Cav1-IRES-GFP, Cav1Y14F-IRES-GFP or IRES-GFP. GFP+ cells were sorted. Cells were stimulated with 10 μg/ml of anti-IgM (Fab‘)2 or with 0.5 mM perV for the indicated times or left unstimulated and further treated as in (c). PLA signals were normalized to the PLA signals of K46-Cav1-GFP cells stimulated for 5 min with 0.5 mM perV for each experiment. At least 500 individual cells were quantified per experimental condition and experiment. (f) Immunoblot of sucrose density gradient fractions of Cav1-GFP-, Cav1Y14F-GFP- and GFP-transfected K46μml cells. The red boxes denote DRM fractions (7–10). Fractions were analyzed for the distribution of IgM, GM1, LYN, Rac1 and Cav1. The charts show the amount of IgM (left) and Rac1 (right) in the DRMs for each stimulatory condition. The middle chart shows the basal distribution of GM1, LYN, Rac1 and Cav1 in DRMs. Quantified data of (d, n=2-9), (e, n=2) or (f, n=2-3) independent experiments were pooled and statistical analysis performed using unpaired Student’s t test. When significant, P values are indicated. *≤ 0.05, **≤ 0.01, ***≤ 0.001, ****≤ 0.0001. Mean ± s.e.m. are shown.
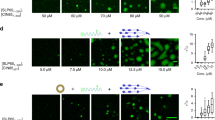
Supplementary Figure 2 Cav1 and the BCR are in close proximity after BCR stimulation.
(a) Basal levels of IgM-BCR expression in splenic CD19+ cells were analyzed by flow cytometry (left panel, n=4-5 animals per genotype). IgM-BCR internalization was assayed by incubating purified B cells from B6.WT or B6.Cav1KO splenocytes with an anti-kappa antibody for 1 hour at 4ºC. Cells were washed once and resuspended in an excess of pre-warmed medium. Samples were taken at the indicated times and mixed with an excess of cold PBS + 0.01% of NaN3. All samples were simultaneously stained with anti-IgM-APC and analyzed by flow cytometry (n=3-4 animals per genotype). IgM-surface levels were normalized to t=0. (b) Basal levels of IgD-BCR expression in splenic CD19+ cells were analyzed by flow cytometry (left panel, n=6-8 animals per genotype). IgD-BCR internalization was assayed as above. Samples were stained with anti-IgD-APC and analyzed by flow cytometry (n=3-4 animals per genotype). (c) Schematics of the BCR(Igα):Cav1 PLA experimental setup. Analysis of the proximity of the BCR to Cav1 was done using an anti-Igα-Fab-coupled PLA oligo and a primary antibody against Cav1 with the corresponding secondary antibody coupled to a PLA oligo. PLA signal is indicated with a red star. (d) PLA controls show single antibodies on unstimulated (top) and 5 min perV stimulated (bottom) on B6.WT splenic B cells. Both secondary antibodies were added. Representative microscope images show DAPI stained nuclei (in blue) and PLA signals (in red). An individual experiment out of 7 independently performed experiments is shown. (e) Cav1-GFP- and GFP-transfected K46μml cells were stimulated with 10 μg/ml of anti-IgM (Fab‘)2 or with 0.5 mM perV for the indicated times or left unstimulated. PLA signals were normalized to the PLA signals of K46-Cav1-GFP cells stimulated for 5 min perV for each experiment. At least 500 individual cells were quantified per experimental condition and experiment. Quantified data of two independent experiments were pooled. Statistical analysis was performed using unpaired Student’s t test. P values were >0.05. Mean ± s.e.m. are shown.
Supplementary Figure 3 The absence of Cav1 reduces B cell responses of Cav1KO mice.
(a) WT and Cav1KO littermates were immunized with NP-Ficoll (T-independent immunization) using aluminum hydroxide as an adjuvant. At day 6 after immunization, splenocytes were analyzed for the frequency of NP-reactive B cells. Representative dot plots and the quantification plotted as mean ± s.e.m. from 5 mice per group are shown. (b) The quantification of NP-reactive Marginal zone (Mz) B cells is plotted as in a. (c) The frequency of proliferating Ki-67+ splenic B cells was analyzed. Representative dot plots and quantification plotted as in (a) are shown. (d) Splenic B cells were isolated and labeled with CFSE. Stimulation was done with IL-4 (50 ng/ml) alone or in combination with 3 μg/ml anti-IgM (Fab’)2 fragments, 1 μg/ml LPS or the cells were left untreated (grey shaded in the representative histogram). After 3 days the proliferation-induced dilution of the CFSE dye was measured by flow cytometry (data are representative of 2 independent experiments). (e) Purified splenic B cells were stimulated with the indicated amounts of anti-IgM (Fab’)2 fragments over night. Cells were then analyzed for CD69 expression by flow cytometry. Quantification shows pooled data from 3 independently performed experiments. (f) Splenocytes were stained with the dye Indo-1. The ratio of Ca2+-bound Indo-1 to Ca2+-unbound Indo-1 was measured by flow cytometry. Baseline was acquired 50 sec, and then 8 μg/ml of anti-IgM (Fab’)2 fragments were added (arrow, n=2). Statistical analysis was performed using unpaired Student’s t test. Wen significant, P values are indicated in each panel. **≤ 0.01, ***≤ 0.001. Mean ± s.e.m. are shown.
Supplementary Figure 4 Increased number of B cells, spontaneous formation of GCs, autoantibodies and shorter lifespan in Cav1KO mice on a mixed background.
(a) The survival curves of WT and Cav1KO littermates are shown. The Log-rank (Mantel-Cox) Test was applied and the P value indicated. (b) The total number of splenocytes in WT and Cav1KO littermates was calculated in adult mice (20 weeks) and in older mice (40 weeks). (c) A representative picture of the spleens used for the quantification shown in b. (d) Relative proportion of B cells in the spleen plotted according to age. (e) The proportion of germinal center (GC) B cells in the spleens of untreated adult mice (<20 weeks) and very old mice (80 weeks) is plotted. (f) The proportion of splenic class-switched IgG1+ B cells is plotted; mice were grouped according to genotype and age (adult mice, <20 weeks, and very old mice 80 weeks). (g) Representative immunofluorescence pictures from the spleen of untreated mice, showing spontaneous proliferation of B cells. (h) The presence of anti-cardiolipin or anti-dsDNA IgG-autoantibodies in the serum of old mice (<50 weeks) was assayed by ELISA. The serum from autoimmune MLR/lpr mice was used as positive control and the serum from AIDKO mice was used as negative control. (i) Representative photomicrograph of the kidneys of old mice (<50 weeks) positively stained for PAS (n=7). (j) Representative immunofluorescence images of the kidneys of old mice (<50 weeks) positively stained for anti-mouse-IgG-FITC (n=5). Each square represents an individual animal. Statistical analysis was performed using Student’s t test. When significant, P values are indicated in each panel. *≤ 0.05, **≤ 0.01, ***≤ 0.001. Mean ± s.e.m. are shown.
Supplementary Figure 5 Increased number of B cells, spontaneous formation of GCs, auto-antibodies and shorter lifespan in mice reconstituted with Cav1KO BM.
(a) Scheme of the repopulation assay. Bone marrow cells obtained from WT or Cav1KO littermates were injected into sub-lethally irradiated recipient Rag2–/–γc–/– mice. Mice were analyzed 25 weeks after injection. (b) The survival curves of the reconstituted mice are shown. The Log-rank (Mantel-Cox) Test was applied and the P value is indicated. (c) Total number of splenocytes in the reconstituted mice (n=3-7). (d) The quantification of the proportion of germinal center (GC) B cells in the spleens of the reconstituted mice and representative dot plots are shown. (e) The presence of anti-cardiolipin or anti-dsDNA IgG autoantibodies in the serum of recipient mice 25 weeks after transplantation was assayed by ELISA (n=3-7). As a negative control, serum from uninjected aged matched Rag2–/–γc–/– mice (Rag) was used. (f) The percent of CD45.1+ and CD45.2+ cells on gated T cells in the WT(CD45.1):B6.CavKO(CD45.2) chimeras (n=4). Naïve (CD44-CD62L+), activated (CD44+CD62L+) and effector memory (CD44+CD62L-) populations in the spleen are shown. (g) Percent of CD45.1+ and CD45.2+ cells on gated CD4+ (left column) or CD8+ (right column) T cells in the lymph nodes (LN) of the WT(CD45.1):B6.CavKO(CD45.2) chimeras (n=4). (h) The percent of CD45.1+ and CD45.2+ cells on gated T cells in the LN of the WT(CD45.1):B6.CavKO(CD45.2) chimeras was analyzed as above. (i) The percent of CD45.1+ and CD45.2+ thymocytes of the WT(CD45.1):B6.CavKO(CD45.2) chimeras are shown. Each square represents an individual animal. Statistical analysis was performed using Student’s t test. When significant, P values are indicated in each panel. *≤ 0.05, **≤ 0.01. Mean ± s.e.m. are shown.
Supplementary Figure 6 Cav1-deficient B cells express a skewed immunoglobulin HC repertoire.
(a) Distribution of the CDR3 length in Igh transcripts of B cells from B6.WT and B6.Cav1KO mice. Fischer’s exact test for long CDR3s (≥15 aa) P=0.0283 (*). (b) The proportion of IgM+λLC+ cells in the BM of the indicated mice was assayed by flow cytometry, n=6-10 (c) Analysis of CD93+ B cells in the spleen. Transitional B cells are identified as CD19+CD93+IgMhigh and anergic B cells as CD19+CD93+IgMlow (n = 3). Each dot represents an individual animal. Statistical analysis was performed using Student’s t test. Mean ± s.e.m. are shown.
Supplementary Figure 7 Increased apoptosis and activation-regulated nanoclustering of the BCR in WEHI cells expressing Cav1.
(a) WEHI immature B cells were lentivirally transfected to express Cav1-IRES-GFP or IRES-GFP. GFP+ cells were sorted and GFP+ stable lines generated. Cells were stimulated with 10 μg/ml of anti-IgM (Fab‘)2 or with 0.5 mM perV for the indicated times or left unstimulated and the IgM:CTxB PLA performed as previously detailed. The resulting signals were normalized to the PLA signals of WEHI-Cav1-GFP cells stimulated for 5 min with perV for each experiment. Quantified data of three independent experiments were pooled and shown as mean ± s.e.m.. (b) Cells were stimulated as in (a) and the BCR(Igα):Cav1 PLA was performed. Quantified data of four independent experiments were pooled and shown as mean ± s.e.m. At least 500 individual cells were quantified per experimental condition and experiment. (c) WEHI immature B cells were lentivirally transfected to express Cav1-IRES-GFP. 24.1% of the cells were GFP+. Cells were cultured for 48 h in the absence (-) or presence of anti-IgM (Fab’)2 fragments. Cell viability was measured by 7-ADD staining and analyzed by flow cytometry. Data of triplicates are shown as mean ± s.e.m.. (d) BM cells were grown ex vivo for 4 days. IL-7 was initially added to the culture. Every 2 days, half of the medium was replaced with medium without IL-7 to ensure the presence of immature B cells. The relative proportion of pre-B cells (B220+IgM–) and immature B cells (B220+IgM+) in the presence of anti-IgM (Fab’)2 fragments was assayed by flow cytometry. (e) The relative MFI of surface IgM from the samples shown in d is plotted as mean ± s.e.m.. Statistical analysis was performed using Student’s t test. When significant, P values are indicated.*≤ 0.05, **≤ 0.01, ***≤ 0.001.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7.
Rights and permissions
About this article
Cite this article
Minguet, S., Kläsener, K., Schaffer, AM. et al. Caveolin-1-dependent nanoscale organization of the BCR regulates B cell tolerance. Nat Immunol 18, 1150–1159 (2017). https://doi.org/10.1038/ni.3813
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3813
This article is cited by
-
Caveolin-1 dolines form a distinct and rapid caveolae-independent mechanoadaptation system
Nature Cell Biology (2023)
-
Overexpression of Toll-like receptor 4 contributes to the internalization and elimination of Escherichia coli in sheep by enhancing caveolae-dependent endocytosis
Journal of Animal Science and Biotechnology (2021)
-
Caveolin1 Tyrosine-14 Phosphorylation: Role in Cellular Responsiveness to Mechanical Cues
The Journal of Membrane Biology (2020)
-
Caveolin-1 is dispensable for early lymphoid development, but plays a role in the maintenance of the mature splenic microenvironment
BMC Research Notes (2018)
-
B cell autoimmunity at the extremes
Nature Immunology (2017)