Abstract
The gene encoding PTEN is one of the most frequently mutated tumor suppressor–encoding genes in human cancer. While PTEN's function in tumor suppression is well established, its relationship to anti-microbial immunity remains unknown. Here we found a pivotal role for PTEN in the induction of type I interferon, the hallmark of antiviral innate immunity, that was independent of the pathway of the kinases PI(3)K and Akt. PTEN controlled the import of IRF3, a master transcription factor responsible for IFN-β production, into the nucleus. We further identified a PTEN-controlled negative phosphorylation site at Ser97 of IRF3 and found that release from this negative regulation via the phosphatase activity of PTEN was essential for the activation of IRF3 and its import into the nucleus. Our study identifies crosstalk between PTEN and IRF3 in tumor suppression and innate immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Li, J. et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 (1997).
Steck, P.A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15, 356–362 (1997).
Myers, M.P. et al. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc. Natl. Acad. Sci. USA 95, 13513–13518 (1998).
Janeway, C.A. Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 (1989).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Honda, K. & Taniguchi, T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6, 644–658 (2006).
Tamura, T., Yanai, H., Savitsky, D. & Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26, 535–584 (2008).
Honda, K., Takaoka, A. & Taniguchi, T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25, 349–360 (2006).
Yu, Y. & Hayward, G.S. The ubiquitin E3 ligase RAUL negatively regulates type i interferon through ubiquitination of the transcription factors IRF7 and IRF3. Immunity 33, 863–877 (2010).
Saitoh, T. et al. Negative regulation of interferon-regulatory factor 3-dependent innate antiviral response by the prolyl isomerase Pin1. Nat. Immunol. 7, 598–605 (2006).
Mori, M. et al. Identification of Ser-386 of interferon regulatory factor 3 as critical target for inducible phosphorylation that determines activation. J. Biol. Chem. 279, 9698–9702 (2004).
Servant, M.J. et al. Identification of the minimal phosphoacceptor site required for in vivo activation of interferon regulatory factor 3 in response to virus and double-stranded RNA. J. Biol. Chem. 278, 9441–9447 (2003).
Lin, R., Heylbroeck, C., Pitha, P.M. & Hiscott, J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18, 2986–2996 (1998).
Fitzgerald, K.A. et al. IKKɛ and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003).
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003).
Di Cristofano, A., Pesce, B., Cordon-Cardo, C. & Pandolfi, P.P. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19, 348–355 (1998).
Myers, M.P. et al. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc. Natl. Acad. Sci. USA 94, 9052–9057 (1997).
Maehama, T. & Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 (1998).
Stambolic, V. et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 (1998).
Hiscott, J. et al. Convergence of the NF-κB and interferon signaling pathways in the regulation of antiviral defense and apoptosis. Ann. NY Acad. Sci. 1010, 237–248 (2003).
Sato, M. et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13, 539–548 (2000).
Wu, J. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2013).
Zhu, M., Fang, T., Li, S., Meng, K. & Guo, D. Bipartite nuclear localization signal controls nuclear import and DNA-binding activity of IFN regulatory factor 3. J. Immunol. 195, 289–297 (2015).
Kumar, K.P., McBride, K.M., Weaver, B.K., Dingwall, C. & Reich, N.C. Regulated nuclear-cytoplasmic localization of interferon regulatory factor 3, a subunit of double-stranded RNA-activated factor 1. Mol. Cell. Biol. 20, 4159–4168 (2000).
Guiducci, C. et al. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J. Exp. Med. 205, 315–322 (2008).
Cao, W. et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat. Immunol. 9, 1157–1164 (2008).
Trotman, L.C. et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128, 141–156 (2007).
Song, M.S., Salmena, L. & Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 13, 283–296 (2012).
Günzl, P. & Schabbauer, G. Recent advances in the genetic analysis of PTEN and PI3K innate immune properties. Immunobiology 213, 759–765 (2008).
Hiscott, J. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 282, 15325–15329 (2007).
Nardozzi, J.D., Lott, K. & Cingolani, G. Phosphorylation meets nuclear import: a review. Cell Commun. Signal. 8, 32 (2010).
Hollander, M.C., Blumenthal, G.M. & Dennis, P.A. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer 11, 289–301 (2011).
Moussavi, M. et al. Oncolysis of prostate cancers induced by vesicular stomatitis virus in PTEN knockout mice. Cancer Res. 70, 1367–1376 (2010).
González-Navajas, J.M., Lee, J., David, M. & Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135 (2012).
Fuertes, M.B. et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J. Exp. Med. 208, 2005–2016 (2011).
Diamond, M.S. et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 208, 1989–2003 (2011).
Chen, X., Wu, D., Zhao, Y., Wong, B.H. & Guo, L. Increasing phosphoproteome coverage and identification of phosphorylation motifs through combination of different HPLC fractionation methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 25–34 (2011).
Acknowledgements
We thank P. Feng (University of Southern California) for Irf3−/−Irf7−/− MEFs; H. Wu (University of California, Los Angeles) for primary Pten+/+ and Pten−/− MEFs; H. Li (Wuhan University) for PtenLoxP/LoxP mice; Y. Zhu and S. Liu (Wuhan University) for plasmid encoding the IL-6 or IL-8 firefly luciferase reporter; X. Chen for help with mass spectrometry; X. Zou and S. Jin for help with statistical analysis; K. Meng and H. Zhang for technical assistance; and X. Fu and Z. Jiang for critical reading and comment on the manuscripts. Supported by the National Basic Research Program of China (2010CB911803), the National Science Foundation of China (81130083 and 31221061) and Hubei Province's Outstanding Medical Academic Leader Program (D.G.).
Author information
Authors and Affiliations
Contributions
S.L. and D.G. designed the experiments and wrote the manuscript.; S.L., M.Z., R.P., T.F., Y.-Y.C. and C.-M.L. performed experimental work; S.L., M.Z., S.C., X.Z., L.G., Y.C., E.J. and D.G. analyzed the data; C.-Q.L., Y.Y. and H.-B.S. provided experimental material; and D.G. conceived of and supervised the study.
Corresponding author
Ethics declarations
Competing interests
D.G. and S.L. are applying for a patent related to PTEN's function in antiviral immunity.
Integrated supplementary information
Supplementary Figure 1 PTEN promotes virus-induced expression of IFNB1 and its downstream genes.
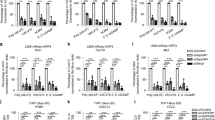
(a) Quantitative RT-PCR analysis of IFNB1 mRNA (left) and ELISA of IFN-β (right) in HEK 293 cells (2 × 105) transfected for 24 h with empty vector (EV) or plasmid for the expression of PTEN, and then untreated (UT) or transfected for another 8 h with poly(I:C) (1 μg/ml). (b, c) Luciferase assay (as in Fig. 1b) (top) and immunoblot analysis of Flag-tagged PTEN and β-actin (below) in HEK293 cells transfected for 24 h with luciferase reporter plasmid for IFN-β (IFN-β-Luc; b) or ISRE (ISRE-Luc; c), plus empty vector or increasing amounts (Wedge: 0, 0.01, 0.05, 0.2 and 1 μg) of plasmid encoding PTEN (below lanes), then left uninfected (UI; left) or infected (right) for 8 h with SeV. (d) Quantitative RT-PCR analysis of IFIT1, ISG15, CXCL10 or RIG-I mRNA in HEK 293 cells (2 × 105) transfected for 24 h with empty vector (EV) or plasmid for the expression of PTEN, and then untreated or transfected for another 8 h with SeV; results were normalized to those of the control gene GAPDH and are presented relative to those of untreated cells transfected with empty vector. (e) Luciferase assay (as in Fig. 1b) in HEK293 cells transfected for 24 h with luciferase reporter plasmid for IL-6 (IL-6-Luc; top) or IL-8 (IL-8-Luc; bottom), plus empty vector (EV) or plasmid encoding PTEN, then left untreated (UT) or infected with SeV or treated with IL-1β (0.02 μg/ml) for 10 h. *p < 0.05; **p < 0.01; ***p < 0.001 (unpaired t-test (a, d, e) or one-way analysis of variant (ANOVA) with post hoc Bonferroni t-test (b, c)). Data are from three independent experiments (a; b, c, top; d, e; mean and s.d. of three independent biological replicates per group in each) or are representative of three independent experiments (b, c, bottom).
Supplementary Figure 2 PTEN deficiency impairs cellular antiviral responses.
(a) Microscopy analyzing VSV replication (left) and immunoblot analysis (right) of VSV-GFP, PTEN and β-actin with the indicated antibodies in HEK293 cells (2 × 105) transfected for 36 h with plasmid encoding non-targeting control siRNA (siControl) or siRNA targeting PTEN (siPTEN1 or siPTEN2), then infected for another 24 h with VSV-GFP (MOI = 0.001). (b) Plaque assay of VSV in HEK293 cells (2 × 105) transfected for 24 h with siRNA-encoding plasmid as in a and left untreated or transfected for 12 h with poly(I:C), then infected or another 24 h with VSV-GFP (MOI = 0.001). (c) Plaque assay of VSV (left) and quantitative RT-PCR analysis of IFNB1 mRNA (right) in 786-O cells stably expressing empty vector (EV) or plasmid encoding wild-type PTEN or PTEN(C124S), and left uninfected or infected for 24 h with VSV-GFP (MOI = 0.01). (d) Cell cytotoxicity assays of PC-3 cells stably expressing empty vector (EV) or plasmid encoding wild-type PTEN or PTEN(C124S), then infected for 24 h with increments of VSV-GFP titers. *p < 0.05; **p < 0.01; ***p < 0.001 (one-way ANOVA (c) or two-way ANOVA with post hoc Bonferroni t-test (b, d)). Data are from three independent experiments (b, c, d; mean and s.d. of three independent biological replicates per group in each) or are representative of three independent experiments (a). BF, bright field.
Supplementary Figure 3 PTEN promotes the induction of IFNB1 independently of Akt pathway.
(a) Breeding scheme for the generation of inducible PTEN-mutant mice and PTEN-wild-type mice. (b) Schematic presentation (left) and PCR analysis (right) of the genotype of mice with respect to the CreER transgene and the target Pten gene by using mice tails as PCR templates. P1, P2, P3 and P4 indicated the primers specific for PtenloxP and Cre. (c) Immunoblotting analysis of PTEN expression in mice spleen from PTEN-wild-type mice and PTEN-mutant mice (line 1, 2 and 3). (d) Luciferase assay (as in Fig. 1b) (top) and immunoblot analysis (below lanes) in HEK 293 cells transfected for 24 h with empty vector (EV) or plasmid encoding PTEN, VHR, PTP1B, Cdc25A, SHP1 or SHIP1, and then left uninfected (UI; left) or infected (right) for 10 h with SeV. (e) Luciferase assay (as in Fig. 1b) in HEK 293 cells transfected for 24 h with empty vector (EV) or plasmid encoding PTEN, and untreated (left half) or treated for 2 h with LY294002 (5 μM each), then left uninfected (UI) or infected for 10 h with SeV. (f) Luciferase assay (as in Fig. 1b) in HEK 293 cells transfected for 24 h with empty vector (EV) or plasmid encoding PTEN, plus empty vector (left half) or plasmid encoding Akt-AA (right top) or Myr-Akt (right bottom), and then left uninfected or infected for 10 h with SeV. Akt-AA, the dominant-negative form of Akt, whose phosphorylation sites (Thr308 and Ser473) are replaced by alanine; Myr-Akt, constitutively active Akt with a myristoylation moiety that results in enhanced plasma membrane binding. (g) Luciferase assay (as in Fig. 1b) in MCF-7 cells (left), HeLa cells (middle) and PC-3 cells (right) transfected for 24 h with empty vector (EV) or plasmid encoding PTEN, and untreated (left half) or treated for 2 h with wortmannin (1 μM each), then left uninfected (UI) or infected for 10 h with SeV. (h) Quantitative RT-PCR analysis (left) of IFNB1 and VSV nucleocapsid (VSV-N) mRNA and immunoblot analysis (right) of Flag-tagged Myr-Akt, Akt-AA and total GAPDH in the livers of wild-type mice (n = 3) with empty vector (EV), plasmid encoding Myr-Akt or Akt-AA delivered to the livers for 9 h by hydrodynamic tail vein injection, infected intrahepaticly with VSV (1 x 107 PFU per mouse), and assessed 6 h after infection. (i) Quantitative RT-PCR analysis (left) of IFNB1 and VSV-N mRNA and immunoblot analysis (right) of total Akt, Akt phosphorylation at Ser473 (p-Akt(S473)) and total GAPDH in the livers of wild-type mice (n = 3) left untreated (UT) or treated intravenously with wortmannin (0.7 mg/kg) or LY294002 (75 mg/kg) for 0.5 h before intrahepatic infection with VSV (1 x 107 PFU per mouse), assessed 6 h after infection. *p < 0.05; **p < 0.01 (one-way ANOVA (d, h, i) or two-way ANOVA with post hoc Bonferroni t-test (e, f, g)). Data are from three independent experiments (d, top; e-g; h, i, left; mean and s.d. of three independent biological replicates per group in each) or are representative of three independent experiments (b, right; c; d, bottom; h, i, right).
Supplementary Figure 4 PTEN promotes IRF3 but not NF-κB-dependent gene transcription.
(a) Quantitative RT-PCR analysis of Isg15, Ifit1, Cxcl10, Ccl5, Cxcl1, Il6, Nfkbia or Nfkb1 mRNA in Pten+/+ MEFs and Pten−/− MEFs left uninfected (UI) or infected for 8 h with SeV. (b) Luciferase assay (as in Fig. 1b) in 293-TLR3 (left), 293 (middle) or 293-TLR4+MD2+CD14+ cells (right) transfected for 36 h with a luciferase reporter plasmid for NF-κB promoter (NF-κB-Luc), plus plasmid encoding non-targeting control siRNA (siControl) or siRNA targeting PTEN, left untreated (UT) or treated with poly(I:C) (10 μg/ml) or LPS (10 μg/ml), or transfected with poly(I:C) (1 μg/ml) for 10 h. *p < 0.05; **p < 0.01 (unpaired t-test (a, b)). Data are from three independent experiments (a, b; mean and s.d. of three independent biological replicates per group in each).
Supplementary Figure 5 Functional screen for the negative phosphorylation site on IRF3 targeted by PTEN.
(a) Luciferase assay (as in Fig. 1b) (top) and immunoblot analysis (below lanes) of IRF3 variants in Irf3−/−Irf7−/− MEFs (1 × 105) transfected with a luciferase reporter plasmid for IFNB1 promoter (IFN-β-Luc), plus empty vector (EV), or plasmid encoding wild-type IRF3 (WT), IRF3 phospho-mimetic or phospho-defective site mutants, left uninfected (UI) or infected for 10 h with SeV. (b) Immunofluorescence microscopy of Irf3−/−Irf7−/− MEFs transfected for 20 h with plasmid encoding wild-type IRF3 (WT) or mutant IRF3 with S97D substitution, left uninfected (UI) or infected for 12 h with SeV, stained with antibody to IRF3 (green) and DAPI (blue). Scale bars, 10 μm. (c) Immunoblot analysis (as in Fig. 5d) of HEK293 cells transfected for 24 h with wild-type IRF3 (WT) or mutant IRF3 with S97A substitution, then left uninfected (–) or infected (+) for 8 h with SeV. (d) Immunoblot analysis of HEK293 cells transfected and infected as in c, followed by nucleus-cytoplasm extraction (and SDS-PAGE of the extracts). (e, f) Luciferase assay (as in Fig. 1b) (top) and immunoblot analysis (below) in Irf3−/−Irf7−/− MEFs transfected for 24 h with luciferase reporter plasmid for IFNB1 (IFN-β-Luc; e) or IFNA4 (IFN-α4; f) promoter, plus plasmid encoding wild-type IRF7 (WT) or mutant IRF7 with the S112A or S112D substitution (top), then left uninfected (UI) or infected for 8 h with SeV. (g) Luciferase assay (as in Fig. 1b) (top) and immunoblot analysis (below) in Pten+/+ MEFs and Pten−/− MEFs transfected for 24 h with luciferase reporter plasmid for IFNB1 (IFN-β-Luc), plus plasmid encoding wild-type IRF7 (WT) or mutant IRF7 with the S112A or S112D substitution. **p < 0.01 (two-way ANOVA with post hoc Bonferroni t-test (g)). Data are from three independent experiments (a (top), e-g (top); mean and s.d. of three independent biological replicates per group in each) or are representative of three independent experiments (a (below lanes), c, d, e-g (bottom)).
Supplementary Figure 6 Identification of phosphorylation of IRF3 at Ser97 in vivo.
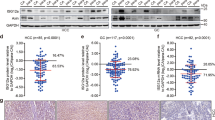
(a) Schematic presentation of IRF3 domain and amino acid sequence with phosphorylation sites identified by mass spectrometry highlighted in red. (b) Tandem mass spectrometry analysis of Ser97-phosphorylated peptide EGLRLAEDRpSK. The observation of fragment ions in the CID product ion spectra was used to localize the phosphorylation site. The presence of the intensive signal at m/z (mass to charge ratio) 628.34 (doubly charged) corresponding to the neutral loss of phosphoric acid (98 Th, doubly charged) due to gas-phase β-elimination from the doubly charged parent ion at m/z 677.34 indicated that the peptide was serine (or threonine)-phosphorylated. The difference of 167 Th between b92+ and b102+ at m/z 520.78 and 604.28 respectively, which corresponded to a phosphoserine residue, was observed in the spectrum. This detection established the phosphorylation site as Ser97 in the sequence. (c, d) The phosphorylation sites stoichiometry (phosphorylation intensity versus total intensity) (c) and Commassie blue staining (d) of recombinant IRF3 (fused with two tags SBP (streptavidin-binding peptide) and CBP (calmodulin-binding peptide)) expressed in 293 cells, purified and treated with GST or GST-PTEN, and then separated by SDS-PAGE and mass spectrometry analysis. (e) ELISA of rabbit immune serum (1), antibody flow-through (2), anti-phospho-Ser97 antibody (3) or anti-nonphospho-Ser97 antibody (4), diluted from 1:500 to 1:106 (right margin), with Ser97 phospho-peptide (left half) and its relative non-phospho peptide (right half) coated as an ELISA plate. (f) Immunoblot analysis of IRF3 with anti-phospho-Ser97 antibody, plus Ser97-phosphorylated peptide (first row) or its relative non-phosphorylation peptide (second row) in Irf3−/−Irf7−/− MEFs transfected with empty vector (EV), wild-type IRF3 (WT) and mutant IRF3 with the S97A substitution (top); below (input), immunoblot analysis with antibody to IRF3 and β-actin. (g) Immunoblot analysis of PC-3 cells infected for 0-8 h with SeV, followed by nucleus-cytoplasm extraction (5% of cytoplasmic extracts and 10% of nuclear extracts separated by SDS-PAGE). Data are representative of three independent experiments (a-g).
Supplementary Figure 7 In vitro and in vivo phosphatase activity of PTEN.
(a) Commassie blue staining of recombinant GST-tagged wild-type PTEN, VHR, PTP1B, Cdc25A and mutant PTEN with the C124S, G129E or G129R substitution expressed in E. coli BL21 (DE3) cells and purified. (b, c) Lipid phosphatase activity assay and serine or threonine phosphatase activity assay of the recombinant proteins as in a, incubated for 2 h with PtdIns-(3,4,5)-P3 (b) or Ser or Thr-phosphorylated peptide (c). (d) In vitro phosphatase assay of GST, recombinant wild-type PTEN, mutant PTEN with the C124S substitution, CIP (Calf intestinal alkaline phosphatase) and λ-PPase (Lambda protein phosphatase) (positive control), incubated for 1 h with recombinant Flag-tagged IRF3 (expressed in HEK293 cells and immunoprecipitated with antibody to Flag). Numbers under lanes indicate IRF3 Ser97 phosphorylation band intensity, normalized to that of total IRF3. (e, f) In vitro phosphatase assay of GST, recombinant wild-type PTEN and CIP, incubated for 1 h with the cell lysates of PC-3 cells left uninfected (–) or infected for 6 h with SeV (+). (g, h) Immunoblot analysis of IRF3 phosphorylated at Ser97 (p-IRF3(S97)) and total IRF3, PTEN and GAPDH (g) and ELISA of IFN-β (h) in PC-3 cells stably expressing empty vector (EV) or plasmid encoding wild-type PTEN and infected for 0-12 h with SeV. Data are from three independent experiments (b, c, h; mean and s.d. of three independent biological replicates per group in each) or are representative of three independent experiments (a, d-g).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–3 (PDF 2579 kb)
Rights and permissions
About this article
Cite this article
Li, S., Zhu, M., Pan, R. et al. The tumor suppressor PTEN has a critical role in antiviral innate immunity. Nat Immunol 17, 241–249 (2016). https://doi.org/10.1038/ni.3311
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3311
This article is cited by
-
Investigating the effects of PTEN mutations on cGAS-STING pathway in glioblastoma tumours
Journal of Neuro-Oncology (2024)
-
Lysine methyltransferase SMYD2 inhibits antiviral innate immunity by promoting IRF3 dephosphorylation
Cell Death & Disease (2023)
-
IDH1 mutation impairs antiviral response and potentiates oncolytic virotherapy in glioma
Nature Communications (2023)
-
The role of TBK1 in cancer pathogenesis and anticancer immunity
Journal of Experimental & Clinical Cancer Research (2022)
-
Transcriptional landscape of PTEN loss in primary prostate cancer
BMC Cancer (2021)