Abstract
The Pim (proviral integration site for Moloney murine leukemia virus) proteins compose a serine threonine kinase family whose members regulate cell proliferation, migration and cell survival. However, whether Pim kinases participate in innate immune responses is unclear. Here, we show for the first time that Pim1 plays an essential role in the production of interferon (IFN)-β by macrophages after their Toll-like receptor (TLR) pathway is activated by pathogen-associated molecular patterns (PAMPs). Specifically, Pim1 was quickly upregulated in an NF-κB-dependent manner after TLR stimulation with PAMPs. Pim1 deficiency reduced TLR3- or TLR4-stimulated IFN-β and IFN-stimulated gene (ISG) expression but not proinflammatory cytokine expression in macrophages. Mechanistically, Pim1 specifically upregulates IRF3 phosphorylation and nuclear translocation. However, this role is not dependent on Pim1 kinase activity. Rather, Pim1 appears to promote IRF3 phosphorylation by enhancing the formation of IFN-β signaling complexes composed of TRIF, TRAF3, TBK1, and IRF3. Poly (I:C)-treated Pim1−/− mice produced less serum IFN-β and were less likely to survive than wild-type mice. These findings show for the first time that Pim1 participates in TLR-mediated IFN-β production, thus revealing a novel target for controlling antiviral innate immune responses.
Similar content being viewed by others
Introduction
Innate immune cells form the first line of host defense against pathogens because they express molecules called pattern recognition receptors (PRRs) on their surface or endosomes. PPRs recognize viral or bacterial molecules called pathogen-associated molecular patterns (PAMPs). Upon binding of PAMPs, PRRs activate multiple signaling cascades that cause innate immune cells to produce inflammatory cytokines1,2,3 and two key subtypes of the type I interferon (IFN) family, namely, IFN-α and IFN-β; the latter is a highly conserved cytokine that plays critical roles in antiviral innate immune responses4,5. Classical examples of PRR-PAMP interactions involve Toll-like receptor 3 (TLR3) and TLR4, which are highly conserved PRRs that recognize double-stranded RNA (dsRNA) and lipopolysaccharide (LPS), respectively6,7. These PRR-PAMP interactions induce the selective recruitment of an immune adaptor protein known as toll-interleukin 1 (IL-1) receptor homology (TIR) domain-containing adaptor inducing IFN-β (TRIF), which binds to the TLR and then recruits downstream signaling molecules that ultimately induce the production of IFN-β8,9,10. Specifically, TRIF forms a signaling complex with TNF receptor-associated factor (TRAF) that activates tank-binding kinase 1 (TBK1)11,12, which in turn phosphorylates the master transcription factor called interferon regulatory factor 3 (IRF3)13,14. This phosphorylation event causes IRF3 to dimerize, translocate into the nucleus, and induce the expression of IFN-β. IFN-β then induces the expression of IFN-stimulated genes (ISGs) through the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway15,16.
Pim is a highly conserved serine/threonine kinase with three isoforms, namely, Pim117,18,19, Pim220 and Pim321. The Pim1 gene contains the upstream CUG start codon, and its Pim1L and Pim1S isoforms are produced by alternative translation18. Pim kinases, which have extensive amino acid homology, are constitutively active and play critical roles in multiple cellular functions22,23, including cell cycle control24, growth25, proliferation26, migration27, apoptosis28,29 and survival30. Indeed, numerous studies have shown that all Pim kinases are oncogenic proteins that promote tumorigenesis via diverse signaling pathways31,32,33. Pim kinases also participate in adaptive immune responses, again via disparate mechanisms34. For example, Pim1 promotes lymphocyte proliferation and survival by suppressing apoptosis35 and promoting NFATc1 activity36. Moreover, it enhances CD8 + T-cell survival, promotes CD8 + T-cell memory, and fosters B-cell proliferation37,38,39.
While Pim2 also promotes B-cell survival, it inhibits T-cell immune responses; this is achieved by downregulating T-cell production of pro-inflammatory cytokines40. In contrast, while Pim3 also inhibits T-cell responses, it accomplishes this by inhibiting CD4 + and CD8 + T-cell proliferation and activation41. In addition, while all Pim kinases are expressed in both Th1 and Th2 cells, their expression is higher in Th1 cells, and they promote Th1-cell differentiation from precursor Th cells42.
In contrast, very little is known about the potential role of the Pim kinase family in innate immune responses. Some very limited recent evidence suggests that these kinases can also participate in this arm of the immune system, especially during viral infection. In particular, the results of two studies suggest that Pim1 may be able to modulate virus-induced type I IFN signaling, which is an important mediator of innate immunity43,44. Here, we expand this finding by conducting exploratory analyses with Pim1-knockout mice and RNA-seq analyses. We show for the first time that Pim1 facilitates the innate immune responses that are driven by TLR-mediated IFN-β production. Specifically, we observed that Pim1 expression was elevated soon after TLR stimulation and that Pim1 deficiency significantly reduced the phosphorylation and nuclear translocation of IRF3. Our other findings suggest that Pim1 promotes IFN-β expression by enhancing the formation of a cell surface signaling complex composed of TRIF, TRAF3, TBK1, and IRF3; this complex then induces IRF3 phosphorylation. Importantly, the kinase activity of Pim1 was not needed for this function. Our findings thus show that Pim1 can positively regulate the TLR signaling pathway. These observations may provide insights into potential approaches to controlling antiviral responses.
Materials and Methods
Mice
Pim1−/− mice were generated by Macrogen via CRISPR/Cas9-mediated genome editing. All mice were on the C57BL/6 genetic background and were bred in the animal facility under specific-pathogen-free conditions. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Ewha Womans University (No. 19-006).
Cells
Murine BMDMs were generated by flushing bone marrow cells from the femurs and tibias of 8- to 10-week-old male C57BL/6 mice, suspending them in Dulbecco’s modified Eagle’s medium (DMEM; HyClone) supplemented with 20% FBS (HyClone), 100 units ml−1 penicillin (HyClone) and 100 μg ml−1 streptomycin (HyClone), and culturing them at 37 °C and 5% CO2 for 1 d. Nonadherent cells were then further cultured with 10 ng ml−1 recombinant human macrophage colony-stimulating factor (R&D Systems) for 7 d. Pim1−/− RAW264.7 cells were generated by ToolGen via CRISPR/Cas9-mediated genome editing. A stable HEK293-TLR3 cell line was kindly provided by Dr. I.H. Choi (Yonsei University College of Medicine, Seoul, South Korea). RAW264.7, HEK293-TLR3 and Plat-E cells were cultured in DMEM supplemented with 10% FBS, 100 units ml−1 penicillin and 100 μg ml−1 streptomycin.
Reagents and antibodies
BAY 11-7082 (#B5556), BAY 11-7085 (#B5681), PD98059 (#P215), SB203580 (#S8307) and SP600125 (#S5567) were obtained from Sigma‒Aldrich, and SMI-4a (#S8005) was obtained from Selleck Chemicals. Antibodies specific for p-TBK1 (#5483, 1:1,000), TBK1 (#3013, 1:1,000), p-IRF3 (#4947, 1:1,000), IRF3 (#4302, 1:1,000), p-STAT1 (#9167, 1:1,000), p-NF-κB p65 (#3033, 1:1,000), NF-κB p65 (#8242, 1:1,000), p-ERK (#4370, 1:1,000), p-p38 (#9215, 1:1,000) and p-JNK (#9251, 1:1,000) were purchased from Cell Signaling Technology. Antibodies specific for Pim1 (#sc-13513, 1:1,000), Pim2 (#sc-13514, 1:1,000), Pim3 (#sc-98959, 1:1,000), HA (#sc-7392, 1:1,000), GST (#sc-138, 1:1,000), GFP (#sc-9996, 1:1,000), β-actin (#sc-87778, 1:1,000) and GAPDH (#sc-87724, 1:1,000) were obtained from Santa Cruz Biotechnology. A TRIF-specific antibody (#NB120-13810, 1:1000) was purchased from Novus Biologicals, while a Flag-specific antibody (#F3156, 1:10,000) was purchased from Sigma‒Aldrich. Horseradish peroxidase-conjugated secondary antibodies (1:10,000) were obtained from The Jackson Laboratory.
Cell stimulation
For TLR stimulation, 1 × 106 BMDMs or RAW264.7 cells were seeded in 12-well plates and then treated with 100 ng ml−1 Pam3CSK4 (InvivoGen), 10 μg ml−1 poly (I:C) (Sigma‒Aldrich) or 100 ng ml−1 LPS (Sigma‒Aldrich) for the indicated times.
Plasmids
The pMX-IRES-EGFP plasmids containing Flag-tagged WT or mutant murine Pim kinase family members and the pIRES-hrGFP-2a plasmid containing HA-tagged murine Pim1 were a gift from Dr. N.S. Kim (Chonnam National University Medical School, Gwangju, Republic of Korea) and have been described previously45. The pCMV6 plasmid containing Flag-tagged human TRIF was obtained from Dr. W.S. Ryu (Yonsei University, Seoul, Republic of Korea). The pcDNA3.1 plasmids containing Flag-tagged human WT TBK1 or TBK1 (K38A) and pEGFP-C1 plasmids containing human WT IRF3, IRF3 (5D) or IRF3 (5 A) were gifts from Dr. J.Y. Lee (Gwangju Institute of Science and Technology, Gwangju, Republic of Korea). The pFlag-CMV2 plasmids containing murine TRAF2, TRAF3, TRAF5 or TRAF6 were previously described46,47. To generate WT GST-fused human IRF3 and its and deletion mutants, the ORF sequence of IRF3 was amplified from pEGFP-C1-IRF3 by site-directed mutagenesis, and the WT and mutant sequences were subcloned into pEBG plasmids.
Cell transfection
For plasmid DNA transfection, HEK293-TLR3 or HEK-293T cells were transfected with the indicated plasmids for 36 h by using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. For siRNA transfection, 1 × 106 BMDMs seeded in 12-well plates were transfected with 20 nM control or murine Pim1-specific siRNA (#AM16708-150114, Ambion) for 36 h by using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s protocol.
Retroviral transduction
To generate retroviruses, supernatants were collected from Plat-E packaging cells that were transfected with the empty pMX-IRES vector or pMX-IRES-Flag-Pim1 (WT, K67M or DN) plasmid and then filtered through a 0.45-μm filter. For retroviral transduction, 1 × 106 BMDMs seeded in 12-well plates were incubated with retroviral supernatants in the presence of 10 μg ml−1 polybrene (Sigma‒Aldrich) for 2 d.
Immunoprecipitation and immunoblot analysis
Whole-cell lysates were obtained by washing cells with cold PBS (HyClone) and lysing them with RIPA buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 0.1% SDS and 0.5% sodium deoxycholate] containing phosphatase and protease inhibitors. The lysates were immunoprecipitated by incubation with the indicated primary antibodies overnight at 4 °C. Thereafter, they were incubated with protein G Sepharose (Millipore) at 4 °C for 1 h with gentle shaking. The immunoprecipitated proteins were washed with lysis buffer, boiled in 2X SDS sample buffer, separated on 10% SDS-polyacrylamide gels and then electrophoretically transferred onto PVDF membranes (Millipore). The membranes were blocked with 5% BSA and incubated with the indicated primary antibodies and the appropriate HRP-conjugated secondary antibodies.
GST pulldown assay
HEK293T cells transfected with the indicated combinations of plasmids were washed with cold PBS and lysed with lysis buffer [20 mM HEPES (pH 7.4), 150 mM NaCl, 150 mM KCl, 10 mM EDTA, 10% glycerol and 1% NP-40] containing phosphatase and protease inhibitors. Whole-cell lysates were incubated with glutathione-Sepharose 4B (GE Healthcare) at 4 °C for 1 h with gentle shaking and then washed with lysis buffer. The proteins were boiled in 2× SDS sample buffer and then subjected to immunoblot analysis as described above.
Cytoplasmic and nuclear fractionation
Cells were lysed with cytoplasmic extraction buffer [10 mM HEPES (pH 7.4), 10 mM KCl, 1.5 mM MgCl2, 0.5 M DTT and 0.1% NP-40] containing protease inhibitors. After centrifugation at 8000 rpm for 5 min at 4 °C, the supernatants were collected as the cytoplasmic fraction and boiled in 6× SDS sample buffer. The pellets were lysed on ice for 30 min in nuclear extraction buffer [5 mM HEPES (pH 7.4), 300 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA and 25% glycerol] containing protease inhibitors. After centrifugation at 14,000 rpm for 30 min at 4 °C, the supernatants were collected as the nuclear fraction and boiled in 6× SDS sample buffer. The cytoplasmic and nuclear fractions were then subjected to immunoblot analysis as described above.
ELISA
Murine IFN-β levels in cell culture supernatants or murine serum were measured by using mouse IFN beta ELISA kits (Abcam) according to the manufacturer’s instructions.
Total RNA extraction and RT‒qPCR
Total RNA extracted from cells using the Monarch Total RNA Miniprep Kit (New England Biolabs) was reverse transcribed to cDNA by using PrimeScript RT Master Mix (Takara) according to the manufacturer’s instructions. RT‒qPCR was performed by using the SensiFAST SYBR HI-ROX kit (Bioline) on the Step-One-Plus Real-Time PCR instrument (Applied Biosystems). Target mRNA expression levels were normalized to β-actin mRNA expression levels. Primer sequences are listed in Supplementary Table 1.
Luciferase assay
HEK293T cells were transiently transfected with the pGL3-IFN-β-luc and pRL-TK Renilla luciferase plasmids along with the indicated plasmids. Luciferase activity was measured 24 h post-transfection and normalized to Renilla luciferase activity by using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions.
Immunofluorescence assay
Cells were fixed with 3.7% paraformaldehyde in PBS for 10 min at room temperature and then permeabilized in 0.2% Triton X-100 in PBS for 10 min at room temperature. After blocking with 1% BSA in PBS for 1 h at room temperature, the cells were stained with an anti-IRF3 antibody (1:500, #NBP2-67741, Novus Biologicals) in PBS overnight at 4 °C and further stained with an Alexa Fluor 488-conjugated goat anti-rabbit antibody (1:1000, Invitrogen) in PBS for 1 h at room temperature. After staining the cells with DAPI for 5 min at room temperature to visualize nuclei, imaging was performed on an ECLIPSE Ts2R fluorescence microscope (Nikon).
Poly (I:C) injection in mice
WT and Pim1−/− male mice (8–10 weeks) were injected i.p. with a combination of 2.5 mg kg−1 poly (I:C) and 1 g kg−1 D-galactosamine diluted in PBS. Serum was obtained for ELISA 6 h post-administration. Survival was monitored every 6 h for 5 d.
RNA-seq analysis
Total RNA was extracted from WT and Pim1−/− BMDMs treated with LPS (100 ng ml−1) for 4 h with a Monarch Total RNA Miniprep Kit according to the manufacturer’s instructions. Total RNA integrity was measured by using an Agilent Technologies 2100 Bioanalyzer (Agilent) to obtain an RNA integrity number. The mRNA library was prepared with the TruSeq Stranded mRNA Library Prep Kit (Illumina) and sequenced on the NovaSeq 6000 system (Illumina) by DNA Link. To identify differentially expressed genes (DEGs), the raw reads from the RNA-seq library were mapped to the reference genome (Rat rn5) with TopHat (v2.0.13) (http://ccb.jhu.edu/software/tophat), and the aligned results were input into Cuffdiff (v2.2.1) (http://cole-trapnell-lab.github.io/cufflinks/papers). For ontological analysis, genes with a > 2-fold increase in expression, a p value of <0.05, and an FDR of <0.1 were selected and subjected to analysis with DAVID (http://david.abcc.ncifcrf.gov). The RNA sequencing data generated during this study are available in GEO (GSE195582) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi).
Statistical analysis
All data are presented as the means ± SDs and represent at least three independent experiments. Groups were compared by two-tailed Student’s t test or one-way analysis of variance. GraphPad Prism 8.0 software was used for data analysis.
Results
TLR stimuli induce Pim1 expression in macrophages
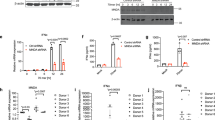
To determine whether Pim kinases are involved in innate immune responses, we stimulated TLR2, TLR3 or TLR4 on bone marrow-derived macrophages (BMDMs) or RAW264.7 macrophages with their specific agonists (Pam3CSK4, poly (I:C) and LPS, respectively) and then examined Pim kinase expression. Both quantitative reverse transcription PCR (RT‒qPCR) and immunoblot analysis showed that all three agonists greatly upregulated the mRNA and protein expression of Pim1 but not Pim2 or Pim3 in both macrophage types (Fig. 1a–d).
a–d Expression of Pim kinases after TLR stimulation. BMDMs a, b, or RAW264.7 cells c, d were treated with the TLR2 activator Pam3CSK4 (100 ng ml−1), TLR3 activator poly (I:C) (10 μg ml−1) or TLR4 activator LPS (100 ng ml−1) for the indicated times, and Pim kinase expression was determined by RT‒qPCR a, c or immunoblot analysis b, d. The yellow arrows in the immunoblots indicate the Pim isoforms (there are two isoforms, three isoforms, and one isoform of Pim1, Pim2 and Pim3, respectively). e–h Time course of LPS-induced Pim kinase and TLR4 downstream gene expression. BMDMs were treated with LPS (100 ng ml−1) for the indicated times. RT‒qPCR was used to determine Pim kinase e, IFN-β f, and IL-6 g expression. h Immunoblot analysis was conducted to determine the expression of the Pim1L and Pim1S isoforms. i–l Effects of inhibiting specific TLR signaling pathways. BMDMs were preincubated with DMSO, the ERK inhibitor PD98059 (10 μM), the p38 inhibitor SB203580 (10 μM), the JNK inhibitor SP600125 (20 μM), or the NF-κB inhibitor BAY 11-7082 (10 μM) or BAY 11-7085 (10 μM) for 30 min and then treated with LPS (100 ng ml−1) i, j or poly (I:C) (10 μg ml−1) k, l for 3 h. Pim1 expression was determined by RT‒qPCR i, k or immunoblot analysis j, l. All mRNA expression values were normalized to β-actin mRNA expression. All data are expressed as the mean ± sd values and are from at least two independent experiments with similar results. *p < 0.05, **p < 0.01 as determined by two-tailed Student’s t test.
There are two major adaptors, that are linked to TLR signaling pathways: TRIF and myeloid differentiation factor 88 (MyD88)1,8,10. To explore the role of Pim1 in these TLR signaling pathways, we largely focused on the Pim1-TLR4 relationship because TLR4 uses both adaptor molecules. However, we also examined the Pim1-TLR3 relationship in preliminary experiments to explore the role of Pim1 in other IFN I-inducing TLR signaling pathways in macrophages. We did not investigate the Pim1-TLR2 relationship further because TLR2 is not known to be a major type I IFN inducer in macrophages1,10,48,49. We first observed that LPS-induced expression of Pim1 started 30 min after LPS stimulation, well before IFN-β or IL-6 expression, which started 1 h after LPS treatment (Fig. 1e–h). It should be noted that LPS induced the expression of both the 44 kDa isoform Pim1L, which is predominantly localized on the cell surface, and the 33 kDa isoform Pim1S, which is mainly localized in the nucleus and cytosol18,31
Since the NF-κB and MAPK signaling pathways are also activated soon after TLR signaling1,5,10, we then asked whether these pathways drive Pim1 expression. When BMDMs were treated with inhibitors of ERK (PD98059), p38 (SB203580), JNK (SP600125) and NF-κB (BAY 11-7082 and BAY 11-7085), only the NF-κB inhibitors significantly reduced LPS-stimulated Pim1 mRNA and protein expression relative to the DMSO control (Fig. 1I, j). This pattern was also observed for poly (I:C)-stimulated Pim1 expression (Fig. 1k, l). Thus, these TLR stimuli appeared to immediately and specifically induce Pim1 expression in macrophages through the NF-κB pathway.
Pim1 deficiency reduces TLR-TRIF-mediated IFN-β production
To explore whether Pim1 participates in TLR signaling, we first knocked down Pim1 expression in BMDMs with small interfering RNA (siRNA) (Supplementary Fig. 1a), stimulated the cells with LPS, and used RT‒qPCR to examine the expression of immune response genes that are activated by LPS. Pim1 knockdown significantly decreased the mRNA and protein expression of antiviral response genes, namely, IFN-β, ISG15, ISG54 and ISG56 (Supplementary Fig. 1b), but had no effect on the expression of proinflammatory (TNF-α, IL-1β, and IL-6) or anti-inflammatory (IL-10) cytokines (Supplementary Fig. 1c). A similar analysis of CRISPR‒Cas9-generated Pim1−/− RAW264.7 cell clones confirmed that Pim1 regulates LPS-stimulated IFN-β and ISG mRNA expression (Supplementary Fig. 2a, b). This effect was also observed when the stimulant was poly (I:C) (Supplementary Fig. 2c, d). Thus, Pim1 may play an important role in TLR4- and TLR3-mediated antiviral gene expression.
To determine whether Pim1 plays a similar role in innate immune responses in vivo, CRISPR‒Cas9 genome editing was used to generate Pim1−/− mice, which have a large deletion (exons I–V) in the Pim1 gene. The Pim1−/− mice were viable, fertile and had normal teeth (data not shown). Flow cytometric analyses indicated that the neutrophil, dendritic cell, and macrophage frequencies in the bone marrow of Pim1−/− mice were comparable to those in wild-type mice, suggesting that Pim1 deletion did not affect the development of innate immune cells (Supplementary Fig. 3). To determine whether in vivo Pim1 deletion also impairs antiviral gene expression in macrophages, we isolated BMDMs from WT and Pim1−/− mice, treated them with LPS, and then performed RNA-seq analysis. Indeed, differential gene expression analysis showed that Pim1 deletion significantly reduced ISG expression (Fig. 2a), while GO and KEGG enrichment analyses showed that the top five terms enriched with genes downregulated in Pim1−/− BMDMs were related to innate immune responses (Fig. 2b, c). Moreover, when Pim1−/− BMDMs were subjected to TLR stimulation (LPS or poly (I:C)), ELISA showed that Pim1−/− BMDMs produced significantly less IFN-β than similarly treated WT BMDMs regardless of the TLR agonist (Fig. 2d, e). Similarly, Pim1−/− BMDMs exhibited drastically reduced mRNA levels of Pim1, IFN-β, ISG15 and ISG56 (Fig. 2f, g) but not of IL-6 and TNF-α (Fig. 2h, i) after LPS or poly (I:C) stimulation when compared to WT cells. It should be noted that deletion of Pim1 did not completely abolish LPS- or poly (I:C)-induced ISG signaling and IFN-β production (Fig. 2d–g). This was also observed when Pim1 was knocked down in BMDMs (Supplementary Fig. 1b). Thus, Pim1 appears to augment TLR4- and TLR3-mediated gene expression rather than be essential for its initiation.
Effect of in vivo Pim1 deletion on the RNA profiles a–c and IFN-β and ISG expression d–i in TLR-stimulated BMDMs. a–c Three WT and three Pim1−/− BMDM replicates were treated with LPS (100 ng ml−1) for 4 h. a Heatmap of the downregulated ISGs in Pim1−/− BMDMs. The selected ISGs are ordered according to their Z score. b GO term enrichment analysis. c KEGG pathway enrichment analysis. The top five pathways are ordered according to their EASE score. d, e ELISA-determined IFN-β levels in the supernatants of WT and Pim1−/− BMDMs treated with LPS (100 ng ml−1) d or poly (I:C) (10 μg ml−1) e for 6 h. f–i RT‒qPCR of Pim1, IFN-β, ISG15, ISG54 f, g, IL-6 and TNF-α h, i in WT and Pim1−/− BMDMs treated with LPS (100 ng ml−1) f, h or poly (I:C) (10 μg ml−1) g, i for the indicated times. mRNA expression values were normalized to β-actin mRNA expression. All data are presented as the mean ± sd values and are from at least two independent experiments with similar results. **p < 0.01 as determined by two-tailed Student’s t test.
Virus-induced IFN-β expression is mediated not only by TLRs but also by two other PPRs, namely, the cytosolic molecules retinoic acid-inducible gene 1 (RIG-I)-like receptor (RLR) and cyclic GMP-AMP synthase (cGAS)4,9. RLR recognizes cytosolic PAMP RNA and induces IRF3 phosphorylation by modulating the adaptor protein mitochondrial antiviral signaling protein (MAVS)50; cGAS recognizes cytosolic PAMP DNA and induces IRF3 phosphorylation by modulating stimulator of interferon genes (STING)51. Recently, Zhang et al. showed that Pim1 downregulates Sendai virus (SeV)-induced IFN-β activation by inhibiting RIG-I-mediated signaling43. This, together with the observation that the TLR-TRIF, RLR-MAVS, and cGAS-STING-mediated signaling pathways share some components, led us to examine the role of Pim1 in RLR and cGAS signaling in BMDMs. Similar to treatment with TLR2, TLR3, and TLR4 agonists (Fig. 1a), treatment with agonists specific for either RLR [poly (I:C) transfection] or cGAS (2′3′-cGAMP transfection) stimulated Pim1 mRNA expression in WT BMDMs (Supplementary Fig. 4a, b, left-hand panels). Moreover, as expected, both stimuli upregulated IFN-β protein production and mRNA expression and IL-6 and TNF-α mRNA expression in WT BMDMs (Supplementary Fig. 4a–d). However, Pim1 deletion showed that Pim1 acted quite differently in RLR and cGAS signaling: Pim1−/−, associated with a further increase in RLR-mediated IFN-β, IL-6, and TNF-α production, had no effect on cGAS-mediated IFN-β production but downregulated cGAS-mediated IL-6 and TNF-α expression (Supplementary Fig. 4a–d). Thus, while Pim1 is upregulated in macrophages by specific agonists of TLR, RLR, and cGAS signaling, it plays quite different roles in the inflammatory outcomes of these pathways: (i) in the TLR pathway, it promotes IFN-β expression but has no effect on IL-6 and TNF-α expression; (ii) in the RLR pathway, it inhibits IFN-β, IL-6 and TNF-α expression; and (iii) in the cGAS pathway, it has no effect on IFN-β expression but increases IL-6 and TNF-α expression. These results suggest that Pim1 may shape the specificity of TLR- and RLR-mediated IFN-β production.
Pim1 promotes TLR4-mediated IFN-β production via the TRIF-TBK1-IRF3 axis in a kinase activity-independent manner
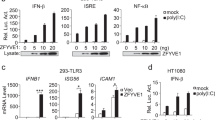
LPS induces IFN-β expression via the TLR4-TRIF pathway: specifically, LPS-bound TLR4 recruits TRIF, which forms a complex with TRAF that activates TBK1, which in turn phosphorylates IRF3 and induces its nuclear translocation and IFN-β transcription9,10,11. To identify the mechanism by which Pim1 regulates IFN-β production, HEK293T cells were transfected with an IFN-β promoter-driven luciferase reporter, the internal control Renilla luciferase reporter, and Flag-tagged Pim1 along with plasmids expressing the upstream kinases that are involved in TLR4-mediated signaling (TRIF, TRAF3 and TBK1). Alternatively, the cells were cotransfected with a plasmid expressing constitutively active IRF3 (5D)52. Compared to the empty vector, the Pim1 expression plasmid significantly increased TRIF-, TBK1-, and 5D-induced IFN-β-driven luciferase activity but not TRAF3-induced IFN-β-driven luciferase activity (Fig. 3a). This suggests that Pim1 regulates IFN-β production through the TRIF-TBK1-IRF3 axis.
a Ability of Pim1 to enhance the IFN-β expression induced by TLR downstream signaling molecules or constitutively active IRF3 (5D). HEK293T cells were transfected for 24 h with an IFN-β promoter-driven luciferase reporter, internal control Renilla luciferase reporter, and Flag-Pim1 plasmid [or its empty vector (EV)] together with plasmids expressing Flag-TRIF, Flag-TRAF3, Flag-TBK1, EGFP-IRF3 (5D) or the corresponding EVs. Luciferase values are presented as fold induction relative to the values in EV-transfected cells. b–f Role of the kinase activity of Pim1 in its ability to promote IFN-β expression. In b, HEK293T cells were transfected with the IFN-β luciferase reporter and EGFP-IRF3 (5D) as described in a along with increasing amounts of the Flag-Pim1 WT or K67M plasmid. The Pim1 K67M mutant lacks kinase activity. Luciferase activity was measured as described in a. In c, BMDMs were preincubated with increasing amounts of the Pim1 kinase inhibitor SMI-4a, treated with LPS (100 ng ml−1) for 3 h, and then subjected to RT‒qPCR analysis of IFN-β. In d, HEK293T cells were transfected with Flag-Pim1 or its K67M or double-negative (DN) mutant along with Flag-TBK1 and were then subjected to RT‒qPCR analysis of IFN-β (left) or immunoblot analysis of the plasmid constructs (right). In e, f, Pim1−/− BMDMs were transduced with retroviral vectors expressing Flag-tagged WT or mutant Pim1 and treated with LPS (100 ng ml−1) for 3 h or 6 h. After 3 h, the cells were subjected to RT‒qPCR analysis of IFN-β (e, left) or immunoblot analysis of Pim1 (e, right). After 6 h, the IFN-β and IL-6 levels in the supernatant were measured with ELISA f. All mRNA expression values were normalized to β-actin mRNA expression. All data are presented as the mean ± sd values and are from at least two independent experiments with similar results. **p < 0.01, ****p < 0.0001 as determined by two-tailed Student’s t test.
To test whether the kinase activity of Pim1 is required for its ability to regulate IRF3, we transfected luciferase reporter-expressing HEK293T cells with IRF3 5D and a kinase-dead Pim1 mutant (K67M)45 instead of WT Pim1. The mutant induced the same level of IFN-β-luciferase activity as WT Pim1 (Fig. 3b). Similarly, coculturing BMDMs with SMI-4a, which inhibits Pim1 kinase activity23, did not affect their LPS-induced IFN-β mRNA levels (Fig. 3c). Moreover, the ability of WT Pim1 to enhance the ability of the TBK1 expression plasmid to induce IFN-β mRNA expression in HEK293T cells was reproduced when WT Pim1 was replaced with the kinase-dead (K67M) or dominant-negative (DN) mutant of Pim1 (Fig. 3d). In addition, Pim1−/− BMDMs that were transduced with retroviral vectors encoding WT, K67M or DN Pim1 produced the same amounts of IFN-β mRNA and protein after LPS treatment (Fig. 3e). In contrast, neither WT Pim1 nor K67M or DN Pim1 enhanced the production of IL-6 in Pim1−/− BMDMs after LPS stimulation (Fig. 3f). Thus, the kinase activity of Pim1 does not appear to be needed for its role in TLR4-mediated IFN-β production.
Pim1 positively regulates IRF3 phosphorylation and nuclear translocation
To determine how Pim1 regulates TLR4-mediated IFN-β expression, we asked whether its deletion affects the phosphorylation of the protein kinases that are involved in TLR4-mediated signaling. Notably, Pim1−/− BMDMs exhibited decreased IRF3 and STAT1 phosphorylation after LPS treatment compared with WT BMDMs, but phosphorylation of the kinases (TBK1, NF-κB, ERK, p38, and JNK) was unaffected (Fig. 4a). Since STAT1 acts downstream and the kinases act upstream of IRF315,16, it appears that Pim1 regulates IRF3 rather than its kinases. This hypothesis was supported by immunoblot analysis of the cytosolic and nuclear fractions of Pim1−/− and WT BMDMs: deletion of Pim1 significantly reduced the LPS-induced nuclear translocation of IRF3 (Fig. 4b). Pim1−/− BMDMs also exhibited noticeably less nuclear IRF3 immunofluorescence after LPS treatment than WT BMDMs (Fig. 4c). In contrast, WT and Pim1−/− BMDMs displayed similar TBK1 and NF-κB p65 immunofluorescence patterns (Supplementary Fig. 5a, b). Thus, Pim1 appears to positively and directly regulate IRF3 phosphorylation in a manner that is independent of its kinase activity.
a Ability of Pim1 to affect LPS-induced phosphorylation of TLR4 upstream kinases or downstream IRF3. WT or Pim1−/− BMDMs were treated with LPS (100 ng ml−1) for the indicated times and were then subjected to immunoblot analysis of phosphorylated TBK1, IRF3, STAT1, NF-κB p65, ERK, p38 and JNK. b, c Ability of Pim1 to promote the nuclear translocation of IRF3. WT and Pim1−/− BMDMs were treated with LPS (100 ng ml−1) for 1 h. The cytoplasmic and nuclear fractions of these cells were isolated and subjected to immunoblot analysis b, or the intact cells were used for immunofluorescence analysis of endogenous IRF3 with DAPI counterstaining of nuclei. Scale bars, 10 μm. All data are from at least two independent experiments with similar results.
Pim1 associates with TRIF, TRAF3, TBK1, and IRF3
To determine precisely how Pim1 regulates IRF3 phosphorylation, we conducted a series of immunoprecipitation and GST pulldown assays to assess the ability of Pim1 to interact with IRF3 and its upstream activators TRIF, TBK1, and TRAF353,54. First, HEK293T cells were transfected with differently tagged Pim1 and IRF3, TRIF, TBK1 or TRAF3 and then subjected to immunoprecipitation assays using tag-specific antibodies. Pim1 coimmunoprecipitated with IRF3, TBK1, TRIF, and TRAF3 (Fig. 5a–d). Second, coimmunoprecipitation experiments were conducted with LPS-stimulated WT BMDMs and an anti-Pim1 antibody to determine whether Pim1 associates with endogenous TRIF, TRAF3, TBK1 or IRF3. Indeed, Pim1 associated with TRIF, TRAF3, TBK1, and IRF3 under these physiological conditions (Fig. 5e).
a–d Ability of Pim1 to interact with IRF3 and its upstream activators. HEK293T cells were transfected with differently tagged Pim1 constructs plus IRF3 a, TBK1 b, TRIF c, or TRAF3 d, and were then incubated with the indicated tag-specific antibody. The immunoprecipitates were then subjected to immunoblot analysis. e Pim1 interacts endogenously with the TRIF-TRAF3-TBK1-IRF3 signaling complex. BMDMs were stimulated with LPS (100 ng ml−1) for 1 h and subjected to immunoprecipitation with an anti-Pim1 antibody. TRIF, TRAF3, TBK1, IRF3 and Pim1 levels were determined by immunoblotting. f Role of Pim1 kinase activity in the interaction between Pim1 and IRF3. HEK293T cells were transfected with GST-tagged IRF3 and Flag-tagged Pim1 WT or mutant plasmids and subjected to a GST pulldown assay. g Ability of Pim1 to form a ternary complex with TRIF and IRF3. HEK293T cells were transfected with differently tagged Pim1, TRIF and IRF3 constructs and were then subjected to an immunoprecipitation assay. h Ability of Pim1 to form a ternary complex with TBK1 and IRF3. WT and Pim1−/− RAW264.7 cells were treated with LPS (100 ng ml−1) for the indicated times, after which immunoprecipitation analysis was conducted with an antibody against endogenous TBK1. i, j Ability of Pim1 to interact with the kinase-inactive TBK1 (K38A) mutant i and the phosphomimetic (5D) and phosphorylation-defective (5 A) mutants of IRF3 j. HEK293T cells were transfected with HA-Pim1 plus WT or kinase-inactive TBK1 i or WT, 5D or 5 A IRF3 j and were then subjected to an immunoprecipitation assay with the indicated antibody. All data are from at least two independent experiments with similar results. IP immunoprecipitation, WCL whole-cell lysate.
Since the kinase activity of Pim1 was not necessary for its induction of IFN-β expression (Fig. 3b–f), we hypothesized that Pim1 kinase activity is not involved in its interaction with IRF3. Indeed, GST pulldown assays with HEK293T cells that were transfected with GST-tagged IRF3 and WT or mutant Pim1 showed that both kinase-dead and dominant-negative Pim1 were pulled down with IRF3 (Fig. 5f). Since IRF3 binds to TRIF after LPS stimulation9, we next asked whether Pim1 promotes TRIF-IRF3 complex formation. Indeed, immunoprecipitation assays in HEK293T cells showed that TRIF and IRF3 interact, that Pim1 forms a ternary complex with TRIF and IRF3, and that Pim1 significantly enhances the TRIF-IRF3 interaction (Fig. 5g).
After IRF3 is recruited to TRIF, TBK1 phosphorylates IRF39. To determine whether Pim1 participates in the LPS-induced formation of the TBK1-IRF3 signaling complex, Pim1−/− and WT RAW264.7 cells were treated with LPS and then subjected to immunoprecipitation with an antibody that recognizes endogenous TBK1. The results showed that soon after LPS treatment (1 h), a ternary complex containing TBK1, IRF3, and Pim1L formed. Moreover, Pim1 deletion markedly abrogated the interaction between TBK1 and IRF3 (Fig. 5h). Since Pim1L is primarily localized on the plasma membrane and Pim1S localizes to both the cytoplasm and nucleus18,31, our finding suggests that Pim1 quickly complexes with TBK1 and IRF3 on the plasma membrane after LPS treatment. We also observed by an immunoprecipitation assay that Pim1 did not interact with kinase-inactive TBK1 (K38A) (Fig. 5i) or with either the phosphomimetic (5D) or phosphorylation-defective (5 A) mutant of IRF3 (Fig. 5j). The latter observations are consistent with the observation that phosphorylation of IRF3 causes it to dissociate from its signaling complex and translocate into the nucleus9,55. Thus, Pim1 appears to form a signaling complex containing TRIF, TRAF3, TBK1, and IRF3 at the cell surface.
Pim1-deficient mice are more susceptible to poly (I:C)-induced death
Since Pim1-deficient BMDMs stimulated with TLR3 or TLR4 produced little IFN-β but had normal inflammatory cytokine levels (Fig. 2f–i), we asked whether Pim1 regulates innate immune responses in vivo. To this end, we injected WT and Pim1−/− mice intraperitoneally with poly (I:C) and D-galactosamine and then monitored the resulting levels of TLR3-induced inflammatory cytokines in the serum and the survival of the mice. Indeed, Pim1−/− mice had lower serum IFN-β levels but normal serum IL-6 levels and a significantly lower survival rate (Fig. 6). This is consistent with the in vitro observations above, namely, that Pim1 is needed for TLR-mediated IFN-β production but not IL-6 production. Since poly (I:C) is a well-known mimic of pathogenic TLR-activating viruses and IFN-β is a key antiviral cytokine, these findings suggest that Pim1 can regulate the antiviral response in vivo.
WT and Pim1−/− mice were challenged with poly (I:C) (2.5 mg kg−1) plus D-galactosamine (1 g kg−1). a Animal survival was monitored every 6 h for 5 d (WT, n = 13; Pim1−/−, n = 14). b, IFN-β and IL-6 levels in the serum of the mice 6 h after injection (WT, n = 11; Pim1−/−, n = 13). The data are presented as the mean ± sd values and are from at least two independent experiments with similar results. *p < 0.05 as determined by using the log-rank (Mantel‒Cox) test; **p < 0.01 as determined by two-tailed Student’s t test.
Discussion
Our in vitro and in vivo experiments suggest that Pim1, which is well known for its oncogenic functions23,31,32,33, is also a novel regulator of an important innate immune antiviral response, namely, TLR-induced ISG expression and IFN-β production. Specifically, we found that Pim1 was quickly upregulated by TLR-mediated signaling in an NF-κB-dependent manner and that Pim1 expression was associated with IFN-β-mediated immune responses but not pro- or anti-inflammatory cytokine responses. We then showed that Pim1 promoted IFN-β production by positively regulating the phosphorylation and nuclear translocation of IRF3. However, it did not do so by phosphorylating IRF3 or by phosphorylating the IRF3 kinase TBK1. It also did not affect the IRF3 upstream adaptor TRAF3, since Pim1 expression did not change the ability of TRAF3 to activate the IFN-β promoter. Rather, Pim1 seemed to act by enhancing the formation of complexes between IRF3, its upstream adaptors TRIF and TRAF3, and its kinase TBK1. Furthermore, the in vitro findings were reproduced in vivo: when Pim1−/− mice were subjected to poly (I:C)-induced lethality, they produced less serum IFN-β (but not less IL-6) and demonstrated poorer survival than WT mice.
A handful of recent studies support the idea that Pim1 can shape innate immune responses to viruses43,44,56,57,58,59,60, although the mechanisms and outcomes vary widely. Specifically, two studies showed that Pim1 inhibits virus propagation by increasing the population of neutrophils that diminish CD8 + T-cell-mediated suppression of viruses59 or by increasing host cell death56. Conversely, four other studies reported that Pim1 promotes virus propagation by facilitating viral entry57 or viral translation58 or, interestingly, by decreasing the IFN-β signaling in host or immune cells (CD4 + T cells, monocytes, and B cells) that is induced by Zika virus44 or Sendai virus43. However, it should be noted that all of these virus-modulating effects of Pim1, including IFN-β downregulation, are mediated by its kinase activity; in contrast, our study showed that the ability of Pim1 to upregulate TLR-induced IFN-β production was independent of its kinase activity.
The observation that Pim1 can both upregulate and downregulate IFN-β production may reflect the ability of Pim1 to shape different IFN-β-stimulating pathways. Thus, while we showed that Pim1 enhances TLR-mediated IFN-β production by complexing with IRF3 and its adaptors and kinase, the results of the Zika and Sendai virus studies suggested that Pim1 inhibits virus-mediated IFN-β promoter activation by phosphorylating a molecule upstream of TBK1 and thereby blocking the RIG-I pathway (however, the RIG-I protein level was not affected)43,44. This is supported by our preliminary analyses of RLR signaling, which indicated that Pim1 downregulated RLR-mediated IFN-β production. Interestingly, our preliminary analyses also showed that Pim1 had no effect on cGAS-mediated IFN-β production. This variable role of Pim1 in IFN-β production was also mirrored by its disparate contributions to proinflammatory cytokine expression after activation of TLRs (no effect), RLR (downregulation), and cGAS (upregulation). Notably, this variable role of Pim1 is reminiscent of the diverse mechanisms by which Pim kinases promote tumorigenesis31,32,33. Thus, although our RLR or cGAS findings remain to be confirmed and extended with analyses of kinase-deficient Pim1, they tentatively suggest that targeting the kinase-independent interaction between Pim1 and the IRF3 signaling complex could help control viral infections. These findings also confirm that although the TLR, RLR, and cGAS pathways regulate IFN-β production via common players, they are tightly and selectively controlled, thereby inducing appropriate antiviral responses. Our study thus suggests that Pim1 may act as a specificity guide in these pathways.
This idea was further supported by our finding that Pim1 can interact with multiple members of the TRAF family, including TRAF2, TRAF5, and TRAF6 (Supplementary Fig. 6a, b). TRAFs are recruited by PPR-activated TRIF, after which they phosphorylate TBK1, which in turn phosphorylates the TRIF in the complex and causes IRF3 to bind to this TRIF9,46. Several studies suggest that TRAF family members contribute to the specificity of the immune response. For example, TRAF2, TRAF5 and TRAF6 are required for TRIF-mediated or MAVS-mediated but not STING-mediated TBK1 activation9, whereas TRAF3 regulates TLR-mediated and RLR-mediated type I IFN responses53,54. The possibility that TRAF3 acts with Pim1 to endow the IRF3 signaling complex with selectivity is further supported by our finding that although Pim1 interacts with TRAF3, it does not increase the ability of TRAF3 to activate the IFN-β promoter in HEK293T cells.
Our finding that Pim1 promotes IRF3 phosphorylation by promoting the association between IRF3 and its upstream kinases rather than by phosphorylating IRF3 is reminiscent of other mechanisms that regulate IFN-β production11,12. For example, GSK3β positively regulates virus-induced IRF3 activation and IFN-β production by promoting the association of TBK1 with IRF3 or the TRAF2-mediated ubiquitination of IRF3 in a kinase-independent manner46,47,61. Notably, our study also showed that Pim1 interacts with TBK1, but Pim1 deletion did not affect the LPS-induced phospho-TBK1 level. This is consistent with the idea that in this setting, Pim1 acts to bring together various players in IFN-β signaling, thereby promoting the selectivity of the signaling complex.
Finally, our study showed that while Pim1 expression enhanced the interaction between TBK1 and IRF3, only the Pim1L isoform complexed with TBK1 and IRF3, although both isoforms were expressed after TLR stimulation. Since Pim1L but not Pim1S contains a proline-rich motif at its N-terminus18,31, this motif may play a key role in the interaction between TBK1 and IRF3. These data indicate that Pim1 isoforms may play different roles in antiviral responses.
In conclusion, this study showed that Pim1 plays a key role in TLR-mediated IFN-β and ISG production. Specifically, after TLR stimulation, Pim1 is upregulated and promotes IFN-β production by promoting the association between IRF3 and its upstream adaptors and kinases. In summary, our study indicates that Pim1 may serve as a crucial positive regulator of innate immune antiviral responses.
References
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Brubaker, S. W., Bonham, K. S., Zanoni, I. & Kagan, J. C. Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 33, 257–290 (2015).
Takeuchi, O. & Akira, S. Innate immunity to virus infection. Immunol. Rev. 227, 75–86 (2009).
Kagan, J. C., Magupalli, V. G. & Wu, H. SMOCs: supramolecular organizing centres that control innate immunity. Nat. Rev. Immunol. 14, 821–826 (2014).
Matsumoto, M. & Seya, T. TLR3: interferon induction by double-stranded RNA including poly(I:C). Adv. Drug. Deliv. Rev. 60, 805–812 (2008).
Kagan, J. C. et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat. Immunol. 9, 361–368 (2008).
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643 (2003).
Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630 (2015).
Fitzgerald, K. A. & Kagan, J. C. Toll-like receptors and the control of immunity. Cell 180, 1044–1066 (2020).
Fitzgerald, K. A. et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003).
McWhirter, S. M. et al. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl Acad. Sci. USA 101, 233–238 (2004).
Hiscott, J. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 282, 15325–15329 (2007).
Yanai, H. et al. Revisiting the role of IRF3 in inflammation and immunity by conditional and specifically targeted gene ablation in mice. Proc. Natl Acad. Sci. USA 115, 5253–5258 (2018).
Schneider, W. M., Chevillotte, M. D. & Rice, C. M. Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 32, 513–545 (2014).
Sheikh, F., Dickensheets, H., Gamero, A. M., Vogel, S. N. & Donnelly, R. P. An essential role for IFN-beta in the induction of IFN-stimulated gene expression by LPS in macrophages. J. Leukoc. Biol. 96, 591–600 (2014).
Jinesh, G. G., Mokkapati, S., Zhu, K. & Morales, E. E. Pim kinase isoforms: devils defending cancer cells from therapeutic and immune attacks. Apoptosis 21, 1203–1213 (2016).
Saris, C. J., Domen, J. & Berns, A. The pim-1 oncogene encodes two related protein-serine/threonine kinases by alternative initiation at AUG and CUG. EMBO J. 10, 655–664 (1991).
Meeker, T. C. et al. Characterization of the human PIM-1 gene: a putative proto-oncogene coding for a tissue specific member of the protein kinase family. Oncogene Res. 1, 87–101 (1987).
Baytel, D., Shalom, S., Madgar, I., Weissenberg, R. & Don, J. The human Pim-2 proto-oncogene and its testicular expression. Biochim. Biophys. Acta 1442, 274–285 (1998).
Fujii, C. et al. Aberrant expression of serine/threonine kinase Pim-3 in hepatocellular carcinoma development and its role in the proliferation of human hepatoma cell lines. Int. J. Cancer 114, 209–218 (2005).
Bachmann, M. & Moroy, T. The serine/threonine kinase Pim-1. Int. J. Biochem. Cell Biol. 37, 726–730 (2005).
Tursynbay, Y. et al. Pim-1 kinase as cancer drug target: an update. Biomed. Rep. 4, 140–146 (2016).
Morishita, D., Katayama, R., Sekimizu, K., Tsuruo, T. & Fujita, N. Pim kinases promote cell cycle progression by phosphorylating and down-regulating p27Kip1 at the transcriptional and posttranscriptional levels. Cancer Res. 68, 5076–5085 (2008).
Beharry, Z. et al. The Pim protein kinases regulate energy metabolism and cell growth. Proc. Natl Acad. Sci. USA 108, 528–533 (2011).
Zhang, Y., Wang, Z., Li, X. & Magnuson, N. S. Pim kinase-dependent inhibition of c-Myc degradation. Oncogene 27, 4809–4819 (2008).
Dang, Y. et al. Proto-oncogene serine/threonine kinase PIM3 promotes cell migration via modulating Rho GTPase signaling. J. Proteome Res. 19, 1298–1309 (2020).
Lilly, M., Sandholm, J., Cooper, J. J., Koskinen, P. J. & Kraft, A. The PIM-1 serine kinase prolongs survival and inhibits apoptosis-related mitochondrial dysfunction in part through a bcl-2-dependent pathway. Oncogene 18, 4022–4031 (1999).
Yan, B. et al. The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J. Biol. Chem. 278, 45358–45367 (2003).
Muraski, J. A. et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat. Med. 13, 1467–1475 (2007).
Hu, X. F. et al. PIM-1-specific mAb suppresses human and mouse tumor growth by decreasing PIM-1 levels, reducing Akt phosphorylation, and activating apoptosis. J. Clin. Invest. 119, 362–375 (2009).
Nawijn, M. C., Alendar, A. & Berns, A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat. Rev. Cancer 11, 23–34 (2011).
Braso-Maristany, F. et al. PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat. Med. 22, 1303–1313 (2016).
Liu, Z., Han, M., Ding, K. & Fu, R. The role of Pim kinase in immunomodulation. Am. J. Cancer Res. 10, 4085–4097 (2020).
Moroy, T., Grzeschiczek, A., Petzold, S. & Hartmann, K. U. Expression of a Pim-1 transgene accelerates lymphoproliferation and inhibits apoptosis in lpr/lpr mice. Proc. Natl Acad. Sci. USA 90, 10734–10738 (1993).
Rainio, E. M., Sandholm, J. & Koskinen, P. J. Cutting edge: transcriptional activity of NFATc1 is enhanced by the Pim-1 kinase. J. Immunol. 168, 1524–1527 (2002).
Peperzak, V., Veraar, E. A., Keller, A. M., Xiao, Y. & Borst, J. The Pim kinase pathway contributes to survival signaling in primed CD8+ T cells upon CD27 costimulation. J. Immunol. 185, 6670–6678 (2010).
Knudson, K. M. et al. NFkappaB-Pim-1-Eomesodermin axis is critical for maintaining CD8 T-cell memory quality. Proc. Natl Acad. Sci. USA 114, E1659–E1667 (2017).
Banerjee, S. et al. EBNA3C augments Pim-1 mediated phosphorylation and degradation of p21 to promote B-cell proliferation. PLoS Pathog. 10, e1004304 (2014).
Daenthanasanmak, A. et al. PIM-2 protein kinase negatively regulates T cell responses in transplantation and tumor immunity. J. Clin. Invest 128, 2787–2801 (2018).
Guo, Q. et al. Immunotherapy for hepatoma using a dual-function vector with both immunostimulatory and pim-3-silencing effects. Mol. Cancer Ther. 13, 1503–1513 (2014).
Tahvanainen, J. et al. Proviral integration site for Moloney murine leukemia virus (PIM) kinases promote human T helper 1 cell differentiation. J. Biol. Chem. 288, 3048–3058 (2013).
Zhang, X. et al. Identification of new type I interferon-stimulated genes and investigation of their involvement in IFN-beta activation. Protein Cell 9, 799–807 (2018).
Zhou, F., Wan, Q., Chen, Y., Chen, S. & He, M. L. PIM1 kinase facilitates Zika virus replication by suppressing host cells’ natural immunity. Signal Transduct. Target. Ther. 6, 207 (2021).
Kim, K., Kim, J. H., Youn, B. U., Jin, H. M. & Kim, N. Pim-1 regulates RANKL-induced osteoclastogenesis via NF-kappaB activation and NFATc1 induction. J. Immunol. 185, 7460–7466 (2010).
Ko, R. et al. Glycogen synthase kinase 3beta regulates antiviral responses of TLR3 via TRAF2-Src axis. J. Immunol. 203, 2990–2999 (2019).
Ko, R., Park, J. H., Ha, H., Choi, Y. & Lee, S. Y. Glycogen synthase kinase 3beta ubiquitination by TRAF6 regulates TLR3-mediated pro-inflammatory cytokine production. Nat. Commun. 6, 6765 (2015).
Barbalat, R., Lau, L., Locksley, R. M. & Barton, G. M. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 10, 1200–1207 (2009).
Oosenbrug, T., van de Graaff, M. J., Haks, M. C., van Kasteren, S. & Ressing, M. E. An alternative model for type I interferon induction downstream of human TLR2. J. Biol. Chem. 295, 14325–14342 (2020).
Rehwinkel, J. & Gack, M. U. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat. Rev. Immunol. 20, 537–551 (2020).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Joung, S. M. et al. Akt contributes to activation of the TRIF-dependent signaling pathways of TLRs by interacting with TANK-binding kinase 1. J. Immunol. 186, 499–507 (2011).
Hacker, H. et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439, 204–207 (2006).
Oganesyan, G. et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439, 208–211 (2006).
Yang, K. et al. Hsp90 regulates activation of interferon regulatory factor 3 and TBK-1 stabilization in Sendai virus-infected cells. Mol. Biol. Cell. 17, 1461–1471 (2006).
de Vries, M., Smithers, N. P., Howarth, P. H., Nawijn, M. C. & Davies, D. E. Inhibition of Pim1 kinase reduces viral replication in primary bronchial epithelial cells. Eur. Respir. J. 45, 1745–1748 (2015).
Park, C. et al. Pim kinase interacts with nonstructural 5A protein and regulates hepatitis C virus entry. J. Virol. 89, 10073–10086 (2015).
Zhou, F. et al. Pim1 impacts enterovirus A71 replication and represents a potential target in antiviral therapy. iScience 19, 715–727 (2019).
Volberding, P. J. et al. Suppressive neutrophils require PIM1 for metabolic fitness and survival during chronic viral infection. Cell rep. 35, 109160 (2021).
Sugasti-Salazar, M., Campos, D., Valdes-Torres, P., Galan-Jurado, P. E. & Gonzalez-Santamaria, J. Targeting host PIM protein kinases reduces mayaro virus replication. Viruses 14, 422 (2022).
Lei, C. Q. et al. Glycogen synthase kinase 3beta regulates IRF3 transcription factor-mediated antiviral response via activation of the kinase TBK1. Immunity 33, 878–889 (2010).
Acknowledgements
We thank N.S. Kim (Chonnam University), W.S. Ryu (Yonsei University), and J.Y. Lee (Catholic University) for sharing cell lines and plasmids. This work was supported by grants from the National Research Foundation of Korea (2021R1A2C3003675 and 2019R1A5A6099645 to S.Y.L.; 2020R1I1A1A01054670 to R.K.) and by a Korea Basic Science Institute National Research Facilities & Equipment Center grant (2019R1A6C1010020).
Author information
Authors and Affiliations
Contributions
R.K. and S.Y.L. designed the experiments and wrote the manuscript. R.K. performed the majority of the experiments and analyzed the data. J.S. performed the animal experiments. H.P. and N.L. performed the experiments. S.Y.L. analyzed the data and provided scientific discussion.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ko, R., Seo, J., Park, H. et al. Pim1 promotes IFN-β production by interacting with IRF3. Exp Mol Med 54, 2092–2103 (2022). https://doi.org/10.1038/s12276-022-00893-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-022-00893-y
This article is cited by
-
Silencing HOXC13 exerts anti-prostate cancer effects by inducing DNA damage and activating cGAS/STING/IRF3 pathway
Journal of Translational Medicine (2023)
-
Essential oil extracted from Quzhou Aurantii Fructus prevents acute liver failure through inhibiting lipopolysaccharide-mediated inflammatory response
Natural Products and Bioprospecting (2023)