Abstract
Most T lymphocytes, including regulatory T cells (Treg cells), differentiate in the thymus. The age-dependent involution of this organ leads to decreasing production of T cells. Here we found that the output of new Treg cells from the thymus decreased substantially more than that of conventional T cells. Peripheral mouse and human Treg cells recirculated back to the thymus, where they constituted a large proportion of the pool of Treg cells and displayed an activated and differentiated phenotype. In the thymus, the recirculating cells exerted their regulatory function by inhibiting interleukin 2 (IL-2)-dependent de novo differentiation of Treg cells. Thus, Treg cell development is controlled by a negative feedback loop in which mature progeny cells return to the thymus and restrain development of precursors of Treg cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Sakaguchi, S., Miyara, M., Costantino, C.M. & Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10, 490–500 (2010).
Josefowicz, S.Z., Lu, L.F. & Rudensky, A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Romagnoli, P., Hudrisier, D. & van Meerwijk, J.P.M. Preferential recognition of self-antigens despite normal thymic deletion of CD4+CD25+ regulatory T cells. J. Immunol. 168, 1644–1648 (2002).
Hsieh, C.S. et al. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity 21, 267–277 (2004).
Chidgey, A., Dudakov, J., Seach, N. & Boyd, R. Impact of niche aging on thymic regeneration and immune reconstitution. Semin. Immunol. 19, 331–340 (2007).
Nikolich-Žugich, J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat. Rev. Immunol. 8, 512–522 (2008).
Yu, W. et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature 400, 682–687 (1999).
Borgulya, P., Kishi, H., Uematsu, Y. & von Boehmer, H. Exclusion and inclusion of alpha and beta T cell receptor alleles. Cell 69, 529–537 (1992).
McCaughtry, T.M., Wilken, M.S. & Hogquist, K.A. Thymic emigration revisited. J. Exp. Med. 204, 2513–2520 (2007).
Liston, A. et al. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc. Natl. Acad. Sci. USA 105, 11903–11908 (2008).
Almeida, A.R. et al. Quorum-sensing in CD4+ T cell homeostasis: a hypothesis and a model. Front Immunol. 3, 125 (2012).
Liston, A. & Gray, D.H. Homeostatic control of regulatory T cell diversity. Nat. Rev. Immunol. 14, 154–165 (2014).
Cuss, S.M. & Green, E.A. Abrogation of CD40–CD154 signaling impedes the homeostasis of thymic resident regulatory T cells by altering the levels of IL-2, but does not affect regulatory T cell development. J. Immunol. 189, 1717–1725 (2012).
Yang, E., Zou, T., Leichner, T.M., Zhang, S.L. & Kambayashi, T. Both retention and recirculation contribute to long-lived regulatory T-cell accumulation in the thymus. Eur. J. Immunol. 44, 2712–2720 (2014).
Attridge, K. & Walker, L.S. Homeostasis and function of regulatory T cells (Tregs) in vivo: lessons from TCR-transgenic Tregs. Immunol. Rev. 259, 23–39 (2014).
Fink, P.J., Swan, K., Turk, G., Moore, M.W. & Carbone, F.R. Both intrathymic and peripheral selection modulate the differential expression of Vβ5 among CD4+ and CD8+ T cells. J. Exp. Med. 176, 1733–1738 (1992).
McMahan, C.J. & Fink, P.J. RAG reexpression and DNA recombination at T cell receptor loci in peripheral CD4+ T cells. Immunity 9, 637–647 (1998).
Papiernik, M., de Moraes, M.L., Pontoux, C., Vasseur, F. & Penit, C. Regulatory CD4 T cells: expression of IL-2Rα chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 10, 371–378 (1998).
Zhan, Y., Corbett, A.J., Brady, J.L., Sutherland, R.M. & Lew, A.M. Delayed rejection of fetal pig pancreas in CD4 cell deficient mice was correlated with residual helper activity. Xenotransplantation 7, 267–274 (2000).
Agus, D.B., Surh, C.D. & Sprent, J. Re-entry of T cells to the adult thymus is restricted to activated T cells. J. Exp. Med. 173, 1039–1046 (1991).
Leng, Q., Nie, Y., Zou, Y. & Chen, J. Elevated CXCL12 expression in the bone marrow of NOD mice is associated with altered T cell and stem cell trafficking and diabetes development. BMC Immunol. 9, 51 (2008).
Bajoghli, B. et al. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell 138, 186–197 (2009).
Joller, N. et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 40, 569–581 (2014).
Lee, H.M. & Hsieh, C.S. Rare development of Foxp3+ thymocytes in the CD4+CD8+ subset. J. Immunol. 183, 2261–2266 (2009).
Pandiyan, P., Zheng, L., Ishihara, S., Reed, J. & Lenardo, M.J. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 8, 1353–1362 (2007).
Cheng, G., Yu, A. & Malek, T.R. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol. Rev. 241, 63–76 (2011).
Lio, C.W. & Hsieh, C.S. A two-step process for thymic regulatory T cell development. Immunity 28, 100–111 (2008).
Kimmig, S. et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J. Exp. Med. 195, 789–794 (2002).
Miyara, M. et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911 (2009).
Dominguez-Villar, M., Baecher-Allan, C.M. & Hafler, D.A. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 17, 673–675 (2011).
Duhen, T., Duhen, R., Lanzavecchia, A., Sallusto, F. & Campbell, D.J. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 119, 4430–4440 (2012).
Bosco, N., Agenes, F., Rolink, A.G. & Ceredig, R. Peripheral T cell lymphopenia and concomitant enrichment in naturally arising regulatory T cells: the case of the pre-Tα gene-deleted mouse. J. Immunol. 177, 5014–5023 (2006).
Romagnoli, P. et al. The thymic niche does not limit development of the naturally diverse population of mouse regulatory T lymphocytes. J. Immunol. 189, 3831–3837 (2012).
Smigiel, K.S. et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 211, 121–136 (2014).
Tai, X. et al. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity 38, 1116–1128 (2013).
Yasunaga, J. et al. Impaired production of naive T lymphocytes in human T-cell leukemia virus type I-infected individuals: its implications in the immunodeficient state. Blood 97, 3177–3183 (2001).
Pilarski, L.M., Mant, M.J., Ruether, B.A. & Belch, A. Severe deficiency of B lymphocytes in peripheral blood from multiple myeloma patients. J. Clin. Invest. 74, 1301–1306 (1984).
Fisson, S. et al. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J. Exp. Med. 198, 737–746 (2003).
Boursalian, T.E., Golob, J., Soper, D.M., Cooper, C.J. & Fink, P.J. Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 5, 418–425 (2004).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Pavlidis, P. & Noble, W.S. Matrix2png: a utility for visualizing matrix data. Bioinformatics 19, 295–296 (2003).
Cabaniols, J.P., Fazilleau, N., Casrouge, A., Kourilsky, P. & Kanellopoulos, J.M. Most α/β T cell receptor diversity is due to terminal deoxynucleotidyl transferase. J. Exp. Med. 194, 1385–1390 (2001).
Fazilleau, N. et al. T cell repertoire diversity is required for relapses in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J. Immunol. 178, 4865–4875 (2007).
Arden, B., Clark, S.P., Kabelitz, D. & Mak, T.W. Mouse T-cell receptor variable gene segment families. Immunogenetics 42, 501–530 (1995).
Acknowledgements
We thank A. Lew (Walter & Eliza Hall Institute of Medical Research) and A. Egle (Salzburg Cancer Research Institute) for GK-transgenic mice; P. Fink (University of Washington, Seattle) for B6 Rag-GFP mice; A. Rudensky (Memorial Sloan-Kettering Cancer Center) and A. Liston (University of Leuven) for Foxp3-Thy-1.1 mice; S. Guerder and J.-C. Guéry for critical reading of the manuscript; F. Auriol and A. Garnier for help in obtaining human thymuses; F.-E. L'Faqihi-Olive and V. Duplan-Eche for technical assistance at the flow-cytometry facility; S. Allart and A. Canivet for technical assistance at the cellular imaging facility of Inserm U1043, Toulouse; the personnel of the Inserm US006 ANEXPLO/CREFRE animal facility for expert animal care; and the GeT-PlaGe and GenoToul bioinformatics platforms Toulouse Midi-Pyrénées for sequencing, computing and storage resources. Supported by the Fondation pour la Recherche Médicale (O.P.J. and J.P.M.v.M.), the IdEx Toulouse (E.A.R. and P.R.), the Région Midi Pyrénées (O.P.J. and J.P.M.v.M.) and the Agence Nationale pour la Recherche (O.P.J.).
Author information
Authors and Affiliations
Contributions
N.T., J.D. and V.A. performed experiments, analyzed data and contributed to writing the manuscript; M.G. and B.B. performed experiments and analyzed data; C.P. performed experiments; B.L. provided clinical samples; N.F., O.P.J. and E.A.R. designed experiments and analyzed data; J.P.M.v.M. and P.R. designed experiments, analyzed the data and wrote the manuscript; and all authors critically reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Gating strategy used to calculate the proportions of GFP− and GFP+ Tconv cells and Treg cells in thymus and spleen from Rag‑GFPFoxp3-Thy-1.1 mice.
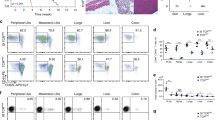
(a) Thymocytes and (b) splenocytes were stained with fluorescent antibodies against the indicated markers and analyzed by flow cytometry. CD4 and CD8 expression on electronically gated live cells (FSC/SSC gate, not shown) is depicted (upper left). CD4+CD8− (CD4SP and CD4+) cells were electronically gated as depicted and Thy1.1 expression was analyzed (upper right). GFP-expression was then analyzed on further gated Thy1.1− Tconv cells (lower left) and Thy1.1+ Treg cells (lower right). Numbers indicate percentages of cells within the indicated electronic gates.
Supplementary Figure 2 Longitudinal analysis of the proportion of splenic Treg cells.
Splenocytes from Rag‑GFPFoxp3-Thy-1.1 mice of indicated ages were stained with fluorescent antibodies against CD4, CD8, and Thy1.1 and analyzed by flow cytometry. Live cells were gated electronically (FSC/SSC gate, not shown) and the proportion of Thy1.1+ Treg cells among CD4+CD8− splenocytes was calculated. Values for all the individual mice analyzed are shown.
Supplementary Figure 3 Analysis of the TCR Vβ repertoire of GFP+ and GFP− Treg cells in the thymus and spleen from Rag-GFPFoxp3-Thy-1.1 mice.
(a) Thymocytes and splenocytes were analyzed as in supplementary Fig. 1 but with the addition of antibodies to the indicated TCR Vβ segments. Expression of Vβ5, Vβ6, and Vβ11 on the indicated, electronically gated CD4+CD8− Thy1.1+ Treg populations is shown. Numbers indicate percentages of cells within indicated electronic gates. Fig. 2c shows the quantitative analysis of thus performed experiments. (b) Percentages of Vβ5-expressing cells in the indicated populations (left). Percentage of peripheral deletion of Vβ5-expressing cells calculated from the data in the left panel (right). The data shown are means ± SD, n=9 from three independent experiments. *p<0.01; Wilcoxon matched pairs signed rank test. (c) Newly developing GFP+ and recirculating GFP− thymic CD4+CD8−Thy-1.1+ Treg cells were analyzed by immunoscope. The peaks within each distribution represent sequences that differ by three bases (one amino acid). The Gaussian-like Vβ-Cβ distribution profiles obtained indicate that the populations of Vβ6- or Vβ11-expressing Treg cells are polyclonal (Vβ5 plots are shown as a control). Shown are representative results from three experiments performed. (d) The percentages of cells expressing the indicated control TCR Vβ segments among the electronically gated indicated CD4+CD8− Thy1.1+ Treg cell populations were analyzed by flow cytometry. The data shown are means ± SD, n=9 from three independent experiments. NS, not significant as determined by the Wilcoxon matched pairs signed rank test.
Supplementary Figure 4 Peripheral depletion of CD4+ T cells and reduced proportions of thymic GFP− Treg cells in GK-transgenic Rag-GFPFoxp3-Thy-1.1 mice.
(a) Flow cytometry of CD4 versus CD8 expression on electronically gated TCRhi splenocytes from control (“Wt”) and GK-transgenic Rag-Gfp/Foxp3-Thy1.1 mice. Numbers indicate percentages of cells within indicated gates. A representative experiment out of three performed is shown. (b) Flow cytometry of CD4 versus CD8 expression by thymocytes from indicated mice. The numbers above the boxed populations indicate the percentages of mature CD4+CD8− cells among total thymocytes. A representative experiment out of three performed is shown. (c) Analysis of Thy-1.1 expression by electronically gated CD4+CD8− thymocytes from indicated mice. Numbers indicate percentage of cells within indicated gates. Quantitative analysis of thus performed experiments is shown in Fig. 2d, left. (d) GFP expression on electronically gated CD4+CD8−Thy-1.1+ thymic Treg cells. Numbers indicate percentage of cells within indicated gates. Quantitative analysis of thus performed experiments is shown in Fig. 2d, right. (e) Cell cycle analysis of GFP+ and GFP− thymic Treg cells in non-transgenic and GK-transgenic mice. Cell cycle status was analyzed by flow-cytometry. Percentages of cycling cells (S, G2, and M phase) for all studied populations are indicated (means ± SD, n=4 control and 4 GK mice). NS, not significant, Mann-Whitney test.
Supplementary Figure 5 Peripheral Treg cells recirculate to the thymus.
(a) In vitro expanded and cell trace violet (CTV)-labeled Treg cells (8–10×106 cells/mouse) were injected i.v. into wt mice. One day later, the thymus of the adoptive host was analyzed by flow-cytometry using antibodies to CD4, CD8, CD25, and Foxp3. In the left plot, expression of Foxp3 and CD25 on electronically gated CD4+CD8− thymocytes is shown. The right plot shows CTV-marked cells in the Treg population, electronically gated as shown. The number indicates the percentage of cells within the indicated gate. Quantitative analysis of thus performed analyses is shown in Fig. 2e. One day after their i.v. injection into wild-type mice, in vitro activated Tconv cells (b) and in vitro generated iTreg cells (c) were found in the spleen and thymus of host mice. Shown are the percentages of indicated cells among Tconv (b) and Treg (c) cells in spleen and thymus (individual mice are shown, horizontal lines indicate mean values, 3 independent experiments, *p<0.01; Wilcoxon matched pairs signed rank test). (d) CXCR4 versus GFP expression by electronically gated CD4+CD8−Foxp3+ thymic Treg cells from Rag-GFP/Foxp3-Thy-1.1 mice. A representative experiment out of three performed is shown. (e) Percentages of Foxp3+ Treg cells among electronically gated CD4+CD8−TCRhiH-2Kb-negative cells from fetal thymus cultures performed in the absence or presence of added in vitro activated H-2Kb-positive Tconv cells. The data from two independent experiments are shown. NS, not significant; Mann Whitney test.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2 (PDF 429 kb)
Rights and permissions
About this article
Cite this article
Thiault, N., Darrigues, J., Adoue, V. et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat Immunol 16, 628–634 (2015). https://doi.org/10.1038/ni.3150
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3150
This article is cited by
-
Attenuation of OX40 signaling suppression by age disrupts peripheral deletion of CD4+ T cells specific for the epidermal autoantigen desmoglein 3
Immunity & Ageing (2023)
-
Cumulative physiological stress is associated with age-related changes to peripheral T lymphocyte subsets in healthy humans
Immunity & Ageing (2023)
-
How autoreactive thymocytes differentiate into regulatory versus effector CD4+ T cells after avoiding clonal deletion
Nature Immunology (2023)
-
Identification of TCR rearrangements specific for genetic alterations in EGFR-mutated non-small cell lung cancer: results from the ADJUVANT-CTONG1104 trial
Cancer Immunology, Immunotherapy (2023)
-
The impact of the gut microbiota on T cell ontogeny in the thymus
Cellular and Molecular Life Sciences (2022)