Abstract
A cytosolic role for the histone methyltransferase Ezh2 in regulating lymphocyte activation has been suggested, but the molecular mechanisms underpinning this extranuclear function have remained unclear. Here we found that Ezh2 regulated the integrin signaling and adhesion dynamics of neutrophils and dendritic cells (DCs). Ezh2 deficiency impaired the integrin-dependent transendothelial migration of innate leukocytes and restricted disease progression in an animal model of multiple sclerosis. Direct methylation of talin, a key regulatory molecule in cell migration, by Ezh2 disrupted the binding of talin to F-actin and thereby promoted the turnover of adhesion structures. This regulatory effect was abolished by targeted disruption of the interactions of Ezh2 with the cytoskeletal-reorganization effector Vav1. Our studies reveal an unforeseen extranuclear function for Ezh2 in regulating adhesion dynamics, with implications for leukocyte migration, immune responses and potentially pathogenic processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Friedl, P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr. Opin. Cell Biol. 16, 14–23 (2004).
Ley, K., Laudanna, C., Cybulsky, M.I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
Pendl, G.G. et al. Immature mouse dendritic cells enter inflamed tissue, a process that requires e- and p-selectin, but not p-selectin glycoprotein ligand 1. Blood 99, 946–956 (2002).
Hamadi, A. et al. Regulation of focal adhesion dynamics and disassembly by phosphorylation of fak at tyrosine 397. J. Cell Sci. 118, 4415–4425 (2005).
Franco, S.J. et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 6, 977–983 (2004).
Huang, C. et al. Talin phosphorylation by cdk5 regulates smurf1-mediated talin head ubiquitylation and cell migration. Nat. Cell Biol. 11, 624–630 (2009).
Zhang, F., Saha, S. & Kashina, A. Arginylation-dependent regulation of a proteolytic product of talin is essential for cell-cell adhesion. J. Cell Biol. 197, 819–836 (2012).
McBride, A.E. in The Enzymes, Protein Methyltransferases Vol. 24 (eds. Clarke, S.G. & Tamanoi, F.) 51–103 (Academic Press, 2006).
Bedford, M.T. & Clarke, S.G. Protein arginine methylation in mammals: Who, what, and why. Mol. Cell 33, 1–13 (2009).
Chuikov, S. et al. Regulation of p53 activity through lysine methylation. Nature 432, 353–360 (2004).
Kouskouti, A., Scheer, E., Staub, A., Tora, L. & Talianidis, I. Gene-specific modulation of taf10 function by set9-mediated methylation. Mol. Cell 14, 175–182 (2004).
Kontaki, H. & Talianidis, I. Lysine methylation regulates e2f1-induced cell death. Mol. Cell 39, 152–160 (2010).
Yang, X.D. et al. Negative regulation of nf-kappab action by set9-mediated lysine methylation of the rela subunit. EMBO J. 28, 1055–1066 (2009).
He, A. et al. Prc2 directly methylates gata4 and represses its transcriptional activity. Genes Dev. 26, 37–42 (2012).
Lee, J.M. et al. Ezh2 generates a methyl degron that is recognized by the dcaf1/ddb1/cul4 e3 ubiquitin ligase complex. Mol. Cell 48, 572–586 (2012).
Egorova, K.S., Olenkina, O.M. & Olenina, L.V. Lysine methylation of nonhistone proteins is a way to regulate their stability and function. Biochemistry 75, 535–548 (2010).
Polevoda, B. & Sherman, F. Methylation of proteins involved in translation. Mol. Microbiol. 65, 590–606 (2007).
Pang, C.N., Gasteiger, E. & Wilkins, M.R. Identification of arginine- and lysine-methylation in the proteome of Saccharomyces cerevisiae and its functional implications. BMC Genomics 11, 92 (2010).
Su, I.H. et al. Polycomb group protein ezh2 controls actin polymerization and cell signaling. Cell 121, 425–436 (2005).
Ezhkova, E. et al. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell 136, 1122–1135 (2009).
Czermin, B. et al. Drosophila enhancer of zeste/esc complexes have a histone h3 methyltransferase activity that marks chromosomal polycomb sites. Cell 111, 185–196 (2002).
Erhardt, S. et al. Consequences of the depletion of zygotic and embryonic enhancer of zeste 2 during preimplantation mouse development. Development 130, 4235–4248 (2003).
Su, I.H. et al. Ezh2 controls B cell development through histone h3 methylation and igh rearrangement. Nat. Immunol. 4, 124–131 (2003).
Kruidenier, L. et al. A selective jumonji h3k27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature 488, 404–408 (2012).
Mukasa, R. et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity 32, 616–627 (2010).
Jain, P., Coisne, C., Enzmann, G., Rottapel, R. & Engelhardt, B. Alpha4beta1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. J. Immunol. 184, 7196–7206 (2010).
Coisne, C., Mao, W. & Engelhardt, B. Cutting edge: Natalizumab blocks adhesion but not initial contact of human T cells to the blood-brain barrier in vivo in an animal model of multiple sclerosis. J. Immunol. 182, 5909–5913 (2009).
Margueron, R. et al. Ezh1 and ezh2 maintain repressive chromatin through different mechanisms. Mol. Cell 32, 503–518 (2008).
García, A.J., Schwarzbauer, J.E. & Boettiger, D. Distinct activation states of alpha5beta1 integrin show differential binding to rgd and synergy domains of fibronectin. Biochemistry 41, 9063–9069 (2002).
Lämmermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the t cell area of the lymph node. Immunity 22, 19–29 (2005).
Yu, D.H., Qu, C.K., Henegariu, O., Lu, X. & Feng, G.S. Protein-tyrosine phosphatase shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 273, 21125–21131 (1998).
Ren, X.D. et al. Focal adhesion kinase suppresses rho activity to promote focal adhesion turnover. J. Cell Sci. 113, 3673–3678 (2000).
García-Bernal, D. et al. Chemokine-induced zap70 kinase-mediated dissociation of the vav1-talin complex activates α4β1 integrin for T cell adhesion. Immunity 31, 953–964 (2009).
Hogg, N., Patzak, I. & Willenbrock, F. The insider's guide to leukocyte integrin signalling and function. Nat. Rev. Immunol. 11, 416–426 (2011).
Goksoy, E. et al. Structural basis for the autoinhibition of talin in regulating integrin activation. Mol. Cell 31, 124–133 (2008).
Critchley, D.R. Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu. Rev. Biophys. 38, 235–254 (2009).
Hobert, O., Jallal, B. & Ullrich, A. Interaction of vav with enx-1, a putative transcriptional regulator of homeobox gene expression. Mol. Cell. Biol. 16, 3066–3073 (1996).
Huang, J. & Berger, S.L. The emerging field of dynamic lysine methylation of non-histone proteins. Curr. Opin. Genet. Dev. 18, 152–158 (2008).
Gingras, A.R. et al. The structure of the c-terminal actin-binding domain of talin. EMBO J. 27, 458–469 (2008).
Giannone, G., Jiang, G., Sutton, D.H., Critchley, D.R. & Sheetz, M.P. Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation. J. Cell Biol. 163, 409–419 (2003).
Ross, T.D. et al. Integrins in mechanotransduction. Curr. Opin. Cell Biol. 25, 613–618 (2013).
Alon, R. & Dustin, M.L. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity 26, 17–27 (2007).
Abram, C.L. & Lowell, C.A. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 27, 339–362 (2009).
Coxon, A. et al. A novel role for the β2 integrin CD11b/CD18 in neutrophil apoptosis: A homeostatic mechanism in inflammation. Immunity 5, 653–666 (1996).
Price, A.A., Cumberbatch, M., Kimber, I. & Ager, A. α6 integrins are required for langerhans cell migration from the epidermis. J. Exp. Med. 186, 1725–1735 (1997).
Tran, A.D. et al. Hdac6 deacetylation of tubulin modulates dynamics of cellular adhesions. J. Cell Sci. 120, 1469–1479 (2007).
Howes, S.C., Alushin, G.M., Shida, T., Nachury, M.V. & Nogales, E. Effects of tubulin acetylation and tubulin acetyltransferase binding on microtubule structure. Mol. Biol. Cell 25, 257–266 (2014).
van der Vlag, J. & Otte, A.P. Transcriptional repression mediated by the human polycomb-group protein eed involves histone deacetylation. Nat. Genet. 23, 474–478 (1999).
Springer, T.A. & Dustin, M.L. Integrin inside-out signaling and the immunological synapse. Curr. Opin. Cell Biol. 24, 107–115 (2012).
Acknowledgements
We thank K. Saijo, C. Schmedt, K. Sadler, P. Dröge, N.E. McCarthy and A. Tarakhovsky for critical reading, discussions and comments on the manuscript; C. Ruedl and K.E. Karjalainen for discussions and suggestions; Y. Zhang (Harvard University) for the EZH2, SUZ12 and EED baculovirus expression vectors; L. Nordenskiold (Nanyang Technological University) for the recombinant octamers; L. Mori and A. Urbano for the EAE-induction protocol; M. Poidinger for processing the microarray data; R. Kraut and A. Matsyik for support for imaging by total internal refraction microscopy; G. White (The University of Manchester) for the eGFP-C1-paxillin plasmid; C.L. Chu (National Taiwan University) for the DC2.4 cell line; and A. Sullivan for editing the manuscript. Supported by the Agency for Science, Technology and Research (BMRC 07/1/21/19/545), the Ministry of Education (MOE2009-T2-1-034 and MOE2013-T2-2-038) and the National Medical Research Council of Singapore (NMRC/CBRG/0057/2014).
Author information
Authors and Affiliations
Contributions
M.G. designed and conducted most of the experiments, interpreted data and was involved in manuscript preparation; N.V. and J.T.L. made major contributions to the manuscript revision; J.T.L. conducted experiments with DCs, neutrophils and Langerhans cells; N.V. and J.F.W. performed the in vitro methyltransferase assays, identified the Ezh2-Vav1 interaction mutant and did experiments with mammary epithelial cells; H.B. performed experiments with OT-II cells; L.Y.J.L. and L.G.N. performed intravital imaging of neutrophils; W.H.N. constructed talin1 expression vectors; N.V. and M.K.L.W. produced the active and inactive Ezh2 (rPCR2) complexes; Y.H.Y. and S.G.S. performed the surface plasmon resonance analysis; T.G. and S.K.S. analyzed the mass spectrometry data; P.C.E.S. and F.G. helped with the in vivo DC subset analysis; S.Y. was involved in setting up the in vitro DC cultures; K.C.C. provided microarray data; A.R.G. provided recombinant talin construct and advice on actin-binding assays; and I.S. designed and interpreted the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
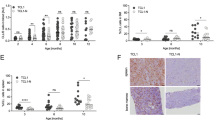
Supplementary Figure 1 Efficient deletion of Ezh2 gene and Ezh2 protein in DCs and neutrophils.
(a) Deletion efficiency of Ezh2 gene in ex vivo isolated DCs. CD11chigh and CD11clow cells were sorted from skin, lymph nodes and spleen of the Ezh2f/Δ and Ezh2f/f Cd11c-Cre mice. RNA was isolated and used for cDNA synthesis followed by specific PCR (full-length Ezh2 transcript: 543 bp, truncated Ezh2 transcript: 246bp). HPRT was used as a loading control. (b) Cell numbers of spinal cord infiltrating leukocytes (top) and percentages of DCs associated with blood vessels (bottom) at the peak of EAE disease (day 21) in Ezh2f/f control and Ezh2f/f Cd11c-Cre mice are shown. Error bars indicate mean ± s.d. of 3 independent experiments. *P=0.01. (c) Deletion efficiency of Ezh2 gene in neutrophils. Gr-1+ granulocytes including neutrophils (Nph) were isolated from bone marrow (BM) and peripheral blood (PB) of poly(I)-poly(C) treated Ezh2f/f and Ezh2f/f Mx1-Cre mice. RNAs from purified cells were processed as described above. (d) Generation of Ezh2-deficient BMDCs in vitro. Bone marrow cells from Ezh2f/f and Ezh2f/f Mx1-Cre mice pre-treated with poly(I)-poly(C) were isolated and cultured in the presence of GM-CSF and IL-4. The phenotypes of the CD11c+ cell populations were analyzed by FACS on day 3, 5 and 6, with or without the final 20 h LPS treatment. Filled gray area indicates the fluorescence content of the isotype control. (e) Deletion efficiency of the floxed Ezh2 locus in BMDCs. RNAs from Ezh2f/f and Ezh2Δ/Δ BM and BMDC were isolated and the deletion efficiency was determined as described in a. (f) Depletion of Ezh2 protein in Ezh2-deficient BMDCs. Expression of Ezh2, Suz12 and Vav1 in nucleus and cytosol was analyzed in BMDCs isolated from culture at day 6 (lane 1, 4) and day 8 with (lane 3, 6) or without LPS (lane 2, 5) stimulated maturation. Expression of lamin B and tubulin was used to control for the purity of the nuclear and cytosolic fractions, respectively.
Supplementary Figure 2 Ezh2-deficient mature DCs display comparable H3K27me3 content and gene-expression profiles.
(a) The content of H3K27me3 of cultured bone marrow derived mature DCs (day 8) from poly(I)-poly(C) treated Ezh2f/f (solid line) and Ezh2f/f Mx1-Cre (Ezh2Δ/Δ) (dashed line) mice were analyzed by intracellular staining (H3K27me3) and flow cytometry. Isotype control is indicated by filled area. Gated mature DCs (CD11c+Class IIhi) are shown in these histograms. (b-d) Gene expression patterns in control and Ezh2-deficeint BMDCs. RNA isolated from control (Ezh2f/f) and Ezh2-deficient (Ezh2Δ/Δ) DCs were processed according to manufacturer’s instruction. Samples were hybridized to Illumina WG6v2 mouse arrays. Raw intensities were extracted using Bead Studio, and quantile normalized. Differential gene expressions were determined with limma, using BH multiple test correction and an FDR cutoff of ≤ 0.05. All data processing and analysis were performed using Accelrys Pipeline Pilot (www.accelrys.com). Biological triplicates were done for each group. (b) Venn diagram showing the overlap in differentially expressed genes (DEGs) between control dendritic cells with versus without LPS stimulation, and Ezh2-deficient DCs with versus without LPS stimulation. Control DC specific DEGs were 29 and Ezh2-deficient DC specific DEGs were 28. In total, 98% of the DEGs (2643) were in common between control and Ezh2-deficient DCs. (c) Scatter plot of log2, normalized intensities from the array of a representative experiment of bone marrow derived control versus Ezh2-deficient immature DCs (iDCs). A Pearson’s r correlation of 0.9947 was obtained. (d) Scatter plot of log2, normalized intensities from the array of a representative sample of control versus Ezh2-deficient LPS-stimulated mature DCs (mDCs). A Pearson’s r correlation of 0.9932 was obtained.
Supplementary Figure 3 Defective migration of Ezh2-deficient leukocytes is not caused by impaired responsiveness to chemoattractant.
(a) Migration of neutrophils on ICAM-1-coated slides in the absence (black bar) or presence (white bar) of fMLP. Control and Ezh2-deficient neutrophils isolated from the bone marrow of poly(I)-poly(C) treated Ezh2f/f and Ezh2f/f Mx-Cre mice were purified using murine neutrophil isolation kit (Miltenyi). Purified neutrophils were seeded to ICAM-1-coated chamber slides and stimulated with fMLP (10-5 M) for 10 minutes in 37ºC. Non-adherent cells were removed, adherent cells were used in parallel for time-lapse imaging at 30-sec interval for 30 min. Phase contrast images were captured using Zeiss inverted microscope at 10x magnification with temperature and CO2 controls. Only cells that were not clustering and could be tracked for the whole time duration were taken into account. Cell tracking was done using Image J software, with Manual Tracking, and Chemotaxis Plugin. About 100 cells from 3 random fields were tracked in each experiment. Statistical analysis of distance was compiled using a “Chemotaxis Plug-in tool” from ImageJ. Error bars represent mean + s.d., ***P=1.1x10-19. (b) Control (solid line filled square) and Ezh2-deficient (dashed line open circle) bone marrow neutrophils were rested at room temperature for 1 hour before pre-warming them at 37ºC for 10 min and stimulated in solution with fMLP for indicated time points. Actin contents were measured by phalloidin staining followed by FACs analysis. (c) Activation of integrin downstream signaling molecules in control and Ezh2-deficient neutrophils. Neutrophils were stimulated as described in b. Equal amount of lysate was resolved with SDS-PAGE, and probed with indicated antibodies. (d) The surface expression of CCR7 on control (blue line) and Ezh2-deficient CD11c+MHCIIhi mature DCs (red line) derived from the bone marrow of poly(I)-poly(C) treated Ezh2f/f and Ezh2f/f Mx-Cre mice were analyzed by flow cytometry. Isotype control is indicated by filled area.
Supplementary Figure 4 Rapid spreading and activation of Ezh2-deficient innate leukocytes.
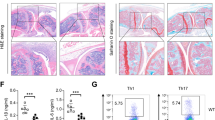
(a) Control (Ezh2f/f) and Ezh2-deficient (Ezh2Δ/Δ) mature DCs derived from poly(I)-poly(C) treated Ezh2f/f and Ezh2f/f Mx1-Cre mice were serum-starved and plated onto RGDS-coated plates for the indicated periods of time. Adherent cells were fixed and stained using crystal violet and DAPI. Data shown are representative of 3 independent experiments with triplicates. The corresponding statistical analysis is shown in Fig. 3d. (b) The surface expression of integrins α5β1, α4ββ1, αXβ2, and αMβ2 in control (blue line) and Ezh2-deficient DCs (red line) were analyzed by flow cytometry. Isotype control is indicated by filled area. Gated mature DCs (CD11c+MHCIIhi) are shown in these histograms. (c) Control and Ezh2-deficient mature BMDCs as in a were plated onto RGDS coated plate for indicated time points. Equal amount of lysate was resolved by the standard SDS-PAGE, and probed with indicated antibodies. “L” indicates cells adherent to poly-L-lysine coated dishes for 45 min. (d) Control (Ezh2f/f) and Ezh2-deficient (Ezh2Δ/Δ) neutrophils isolated from the BM of poly(I)-poly(C) treated Ezh2f/f and Ezh2f/f Mx1-Cre mice were rested as described in Supplementary Fig. 3b and plated onto ICAM-1 coated dishes for indicated time points. Activation of indicated molecules was determined by specific antibodies. (e) Control and Ezh2-deficient mature DC as in a adherent to VCAM-1-coated slide were stained for talin1 (red) and statistical analysis of various adhesion structures (bottom) in control (filled bars) and Ezh2-deficient DCs (open bars) were performed. Nascent adhesions (NAs), focal complexes (FCs), focal adhesions (FAs) and fibrillar adhesions (FBs) were measured by setting the size limits of the measured particles from 0.05-200 µm2. The classifications are as follows: NA <0.25 µm2, FC <0.5 µm2, FA <1.5 µm2, FB > 5 µm2. Error bars represent Mean ± s.e.m. of cells pooled from 3 independent experiments. (Two tailed student’s t test, *p=0.041 (left), 0.008, ***p=0.0002 (left), 5x10-8). (f) The expression of Paxillin, p-Paxillin (Y118) or overall phosphorylated protein (Py99) in control and Ezh2-deficeint DCs on fibronectin coated slides was determined by immunofluorescence staining and the images were acquired either with confocal or TIRF microscopy. (g) Immunofluorescence staining of indicated proteins in control and Ezh2-deficeint neutrophils on ICAM-1 coated slides. Paxillin (red), p-Paxillin (Y118) (green), DAPI (blue). Data shown in this figure are either representative from 1 of 3 (a, b, e (top), f) or 2 (g, d with 3 mice per group) independent experiments or summary (e, bottom) of data acquired from 3 mice per group. Scale bars in e, f, g represent 10 µm.
Supplementary Figure 5 Substitutions of Lys241 and Lys245 in Ezh2 disrupt interactions of Ezh2 with Vav1.
(a) Ezh2–Vav1 interaction domains, Ezh2 (Ezh2201-252) and Vav1 (Vav11-172) with C-terminal His-tag or N-terminal GST-tag, respectively, were purified from E. coli. Mutations of crucial residues (through computational prediction) for this interaction were introduced into Ezh2201-252 and the interactions of these mutated recombinant proteins with Vav1 were tested in His pull down experiments. Ezh2201-252K241/245A mutant was referred to as Ezh2VavMT in the main text. The enzymatic activity of Ezh2 is not affected by this mutation (Venkatesan et al., unpublished data). (b) Methylation of endogenous talin1 from cells expressing wild-type Ezh2 (Ezh2), hyperactive Ezh2 (Ezh2Y641F) or Vav interaction mutant Ezh2 (Ezh2VavMT) were determined as described in Fig. 5g. Data shown in this figure are representative from one of 3 independent experiments. (c) The effects of Vav interaction mutant Ezh2 on its co-localization with focal adhesions were determined by transfecting indicated Ezh2 and mCherry-paxillin constructs into MCF10a cells and the fluorescence signals were measured by confocal microscopy. White boxes indicate the regions enlarged in the following panels (box 1 middle panel, box 2 lower panel). Scar bars, 20 µm.
Supplementary Figure 6 Ezh2 mediates trimethylation of talin1 at Lys2454.
ETD-MS/MS spectra were recorded by Orbitrap Elite in the supplemental activation ETD mode. MS/MS spectra of the unmodified peptide (Red) and the K15Me3 modified peptides (Blue) were displayed in the stack plot with Y-offset of 10. The supplemental activation ETD fragmentation of peptide produced mainly c and z· ions, with some b and y ions. The ions of the small fragments of the two peptides that do not contain the K15Me3 (talin1-K2454) have the same masses (m/z) and were labeled in black. The major c and z· ions of larger fragments that contain the K15 (Red) from the unmodified peptide or K15Me3 (Blue) from the modified peptide were labeled. The spectra showed a systematic 42 Da shift in the fragment containing the K15Me3 modification. The K15 modification site is sandwiched by the series of c and z· ions, indicating unambiguous assignment of the K15Me3 modification. Both peptides have N22 deamidation that are commonly generated during peptide synthesis or sample handling steps. The detailed fragment ions assignment of the supplemental activation ETD-MS/MS spectrum of the unmodified and methylated peptide by Mascot search engine version 2.4 was shown in Supplementary Tables 1 and 2. As a mixture of unmodified, oxidized, deamidated, and/or K15-trimethylated peptides were generated in the in-vitro methylation process, it is impractical to use extracted ion chromatogram method to integrate peak areas of various modified peptides for label-free quantitation. Spectral counting method was used to determine the K15-trimethylation efficiency by the Ezh2. We counted all MS/MS spectra matched to the peptides with no modification, methionine oxidation, deamidation and/or trimethylation. In total, there are 81 MS/MS spectra matched to the peptides. Among them, 31 MS/MS spectra were identified with trimethylation. In summary, the methylation efficiency of the Ezh2 on this peptide is determined to be 37% (details see corresponding Source Data file).
Supplementary Figure 7 Trimethylation of talin1 at Lys2454 is critical for binding to F-actin.
(a) Recombinant talin1(2300-2451) containing C-terminal actin-binding site and dimerization domain was purified and incubated with F-actin. Binding of the recombinant talin1(2300-2451) (wild-type or with mutations at position K2454) to F-actin was determined using the actin-co-sedimentation assays with 4 µM of talin1(2300-2541) and indicated amounts of F-actin; talin1 and actin (1 µM) input (In), co-sedimented talin and actin (Pellet), remaining talin1(2300-2541) in supernatant (Supernatant). Representative images from 1 of 3 independent experiments are shown. (b) Quantitative analysis of the binding of talin1 mutants to various concentrations of F-actin was determined by actin co-sedimentation assay. Means ± s.d. of 3-5 independent experiments are shown, talin1(2300-2541)-K2454 (black squares), talin1(2300-2541)-K2454Q (blue diamonds), talin1(2300-2541)-K2454F (red triangles), talin1(2300-2541)-D2447N (filled purple circle), talin1(2300-2541)-V2444D (open green circle). (Two-way ANOVA with replication). ***P=1.8x10-8 (top), 1.2x10-11 (bottom). (c) The dissociation rate of F-actin from various talin1(2300-2451) was analyzed using Surface plasmon resonance (SPR) measurement as described in materials and methods. Dissociation response for wild-type talin1(2300-2541)-K2454/F-actin at 4, 20 and 40 µM was analyzed, and globally fit rate of dissociation was kd=1.4x10-5 s-1 (second panel from top). Dissociation response for talin1(2300-2541)-K2454Q/F-actin at 4, 20 and 40 µM was analyzed, and globally fit rate of dissociation was kd=4.14x10-6 s-1 (P < 0.0001 against wild-type talin1(2300-2541)-K2454) (third panel from top). Dissociation response for talin1(2300-2541)-K2454F/F-actin complex was not detectable at 4 µM, but consistently measurable at 10, 20 and 40 µM, and globally fit rate of dissociation was kd = 1.4 x 10-5 s-1 (P=0.09 against wild-type talin1(2300-2541)-K2454) (bottom panel). A typical sensorgram showing overlay responses of complex concentrations from initial capturing followed by dissociation (top panel). The initial capturing phase of other graphs was shortened to improve clarity. (d) Biotin labeled dimeric talin1 peptides (aa2450-2461) containing tri-methylated (open bars) or un-modified K2454 (filled bars) were incubated with various concentrations of F-actin and analyzed for their co-sedimentation as described in Fig. 7d except that the co-sedimentated peptides were visualized on dot blots by streptavidin-HRP. Error bars represent the mean ± s.d. of 3 independent experiments. *P=0.026 (left), 0.013 (right). (e-g) The talin1-K2454me3 antibody was generated by immunizing rabbits with (Ac-SEAMK(Me)3RLQAAG)2-K-C-amide branched peptides conjugated to carrier protein followed by affinity depletion and purification (YenZym antibodies LLC.). The specificity of this talin1-K2454me3 antibody was verified by dot-blotting of non-methylated and tri-methylated peptides (e), immunoblotting (IB) of endogenous talin1 (Endo-talin1) using tri-methylated talin1-K2454 peptide-blocking (1 µg/ml) as negative controls (f), and recombinant GFP-talin1 fusion protein using GFP-talin1-2454 mutants as negative controls (g).
Supplementary Figure 8 Ezh2 regulates integrin-dependent, but not integrin-independent, adhesion and migration of leukocytes.
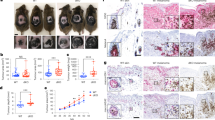
(a) Cervical lymph node cells were prepared from Ezh2f/f and Ezh2f/f Cd11c-Cre mice 24h after FITC painting onto the dorsum of ears. CD11c+FITC+ DCs were analyzed by FACS. Representative plots (left) and percentages of FITC-positive DCs from 7 pairs of mice (right) are shown. Red line indicates the average percentages of FITC+ cells in control and Ezh2-deficient mice. (b) Control and Ezh2-deficient mature bone marrow derived DCs and BM neutrophils isolated from poly(I)-poly(C) treated Ezh2f/f and Ezh2f/f Mx1-Cre mice were plated on poly-L-lysine coated slides for 1 h and F-actin was visualized by phalloidin. F-actin (green), talin1 (red), DAPI (blue) Data shown are representative from 1 of the 2 independent experiments. Scale bars, 10 µm. (c) Epidermal Langerhans cells (LCs) from control Ezh2f/f and Ezh2f/f Cd11c-Cre mice were identified by MHCII staining in steady-state and upon oxazolone stimulations. Representative images of more than 3 independent experiments are shown. Left to right, ***p=6.6x10-5, 8.5x10-5. Scale bars, 100 µm. (d) Migratory LCs (CD11c+MHCIIhiEpCAM+CD103-) in skin draining lymph nodes from control Ezh2f/f and Ezh2f/f; Cd11c-Cre mice were determined by FACS and cell numbers were calculated. *p=0.01, **p=0.002 (e) LCs as in c isolated from epidermis were plated on laminin coated slides for 2 h and identified by MHCII staining. The adhesion structures were determined by anti-talin1 antibody. Cell area was quantified from images. Thirty cells per mouse were scored. ***p=1.3x10-5. Scale bars, 5 µm. (f) Various adhesion structures in control and Ezh2-deficient LCs were quantified according to the criteria specified in Supplementary Fig. 4e. Forty cells per mouse were scored. Error bars in the figures c-f represent mean ± s.e.m. of 3 independent experiments with 3 mice per group (two-tailed student’s t-test with equal variance). Left to right, ***p=1.8x10-7, 7.0x10-6, 1.5x10-5. (g) Migration of control (filled bars) and Ezh2-deficient mature BMDCs (open bars) as defined in b was determined on surfaces coated with various concentrations of fibronectin. Time-lapse images were taken every 5 min for 2 h. Error bars in the figure represent mean ± s.e.m. of around 169-300 cells pooled from 2-3 independent experiments. ***p=2.5x10-18. (h) Migration of control (filled bars) and Ezh2-deficient DCs (open bars) was determined as described in g except some cells were treated with ROCK inhibitor Y27632 (Y27, 30 µM) or myosin II inhibitor blebbistatin (ble, 10 µM) while imaging. ***p=3.6x10-13. (i) Control (filled bars) and Ezh2-deficient mature BMDCs (open bars) were allowed to adherent to slides coated with indicated concentrations of fibronectin for 2 h. Cell areas were calculated using ImageJ. Error bars in h and i represent mean ± s.e.m. of around 200 cells polled from 2 independent experiments. ***p=3.6x10-19. (j) Representative images from i are shown. Control (Ezh2f/f) and Ezh2-deficient (Ezh2Δ/Δ) mature BMDCs were cultured on surfaces coated with different concentrations of fibronectin (FN) in the presence or absence of Y27632. Focal adhesions were visualized by anti-paxillin staining (red), F-actin by Alexa Fluor 488® phalloidin and nucleus by DAPI (blue). Scale bars, 10 µm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1 and 2 (PDF 14400 kb)
Rights and permissions
About this article
Cite this article
Gunawan, M., Venkatesan, N., Loh, J. et al. The methyltransferase Ezh2 controls cell adhesion and migration through direct methylation of the extranuclear regulatory protein talin. Nat Immunol 16, 505–516 (2015). https://doi.org/10.1038/ni.3125
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3125
This article is cited by
-
Lysine methylation promotes NFAT5 activation and determines temozolomide efficacy in glioblastoma
Nature Communications (2023)
-
EZH2 inhibitor Tazemetostat synergizes with JQ-1 in esophageal cancer by inhibiting c-Myc signaling pathway
Medical Oncology (2023)
-
Transient Polycomb activity represses developmental genes in growing oocytes
Clinical Epigenetics (2022)
-
Link between the EZH2 noncanonical pathway and microtubule organization center polarization during early T lymphopoiesis
Scientific Reports (2022)
-
Progranulin regulates the development and function of NKT2 cells through EZH2 and PLZF
Cell Death & Differentiation (2022)