Abstract
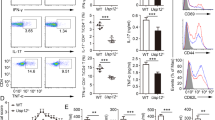
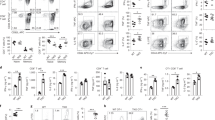
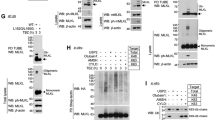
The protease activity of the paracaspase MALT1 is central to lymphocyte activation and lymphomagenesis, but how this activity is controlled remains unknown. Here we identify a monoubiquitination of MALT1 on Lys644 that activated the protease function of MALT1. Monoubiquitinated MALT1 had enhanced protease activity, whereas a ubiquitination-deficient MALT1 mutant with replacement of that lysine with arginine (MALT1(K644R)) had less protease activity, which correlated with impaired induction of interleukin 2 (IL-2) via the T cell antigen receptor in activated T cells. Expression of MALT1(K644R) diminished the survival of cells derived from diffuse large B cell lymphoma of the activated B cell–like subtype (ABC DLBCL), which require constitutive protease activity of MALT1 for survival. Thus, monoubiquitination of MALT1 is essential for its catalytic activation and is therefore a potential target for the treatment of ABC-DLBCL and for immunomodulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Thome, M., Charton, J.E., Pelzer, C. & Hailfinger, S. Antigen receptor signaling to NF-kappaB via CARMA1, BCL10, and MALT1. Cold Spring Harb. Perspect. Biol. 2, a003004 (2010).
Rosebeck, S., Lucas, P.C. & McAllister-Lucas, L.M. Protease activity of the API2-MALT1 fusion oncoprotein in MALT lymphoma development and treatment. Future Oncol. 7, 613–617 (2011).
Staal, J., Bekaert, T. & Beyaert, R. Regulation of NF-κB signaling by caspases and MALT1 paracaspase. Cell Res. 21, 40–54 (2011).
Oeckinghaus, A. et al. Malt1 ubiquitination triggers NF-κB signaling upon T-cell activation. EMBO J. 26, 4634–4645 (2007).
Sun, L., Deng, L., Ea, C.K., Xia, Z.P. & Chen, Z.J. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14, 289–301 (2004).
Wu, C.J. & Ashwell, J.D. NEMO recognition of ubiquitinated Bcl10 is required for T cell receptor-mediated NF-κB activation. Proc. Natl. Acad. Sci. USA 105, 3023–3028 (2008).
Häcker, H. & Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, re13 (2006).
Li, Q. & Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2, 725–734 (2002).
Düwel, M. et al. A20 negatively regulates T cell receptor signaling to NF-κB by cleaving Malt1 ubiquitin chains. J. Immunol. 182, 7718–7728 (2009).
Rebeaud, F. et al. The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat. Immunol. 9, 272–281 (2008).
Hailfinger, S. et al. Malt1-dependent RelB cleavage promotes canonical NF-κB activation in lymphocytes and lymphoma cell lines. Proc. Natl. Acad. Sci. USA 108, 14596–14601 (2011).
Weih, F. et al. Both multiorgan inflammation and myeloid hyperplasia in RelB-deficient mice are T cell dependent. J. Immunol. 157, 3974–3979 (1996).
Marienfeld, R. et al. Signal-specific and phosphorylation-dependent RelB degradation: a potential mechanism of NF-κB control. Oncogene 20, 8142–8147 (2001).
Coornaert, B. et al. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-κB inhibitor A20. Nat. Immunol. 9, 263–271 (2008).
Staal, J. et al. T-cell receptor-induced JNK activation requires proteolytic inactivation of CYLD by MALT1. EMBO J. 30, 1742–1752 (2011).
Hailfinger, S. et al. Essential role of MALT1 protease activity in activated B cell-like diffuse large B-cell lymphoma. Proc. Natl. Acad. Sci. USA 106, 19946–19951 (2009).
Ferch, U. et al. Inhibition of MALT1 protease activity is selectively toxic for activated B cell-like diffuse large B cell lymphoma cells. J. Exp. Med. 206, 2313–2320 (2009).
Staudt, L.M. Oncogenic activation of NF-κB. Cold Spring Harb. Perspect. Biol. 2, a000109 (2010).
Kingeter, L.M. & Schaefer, B.C. Malt1 and cIAP2-Malt1 as effectors of NF-κB activation: kissing cousins or distant relatives? Cell. Signal. 22, 9–22 (2010).
Rosebeck, S. et al. Cleavage of NIK by the API2-MALT1 fusion oncoprotein leads to noncanonical NF-κB activation. Science 331, 468–472 (2011).
Hicke, L. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2, 195–201 (2001).
Pickart, C.M. & Fushman, D. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 8, 610–616 (2004).
Wertz, I.E. & Dixit, V.M. Signaling to NF-κB: regulation by ubiquitination. Cold Spring Harb. Perspect. Biol. 2, a003350 (2010).
Iwai, K. & Tokunaga, F. Linear polyubiquitination: a new regulator of NF-κB activation. EMBO Rep. 10, 706–713 (2009).
Laussmann, M.A. et al. Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8. Cell Death Differ. 18, 1584–1597 (2011).
Rehm, M. et al. Single-cell fluorescence resonance energy transfer analysis demonstrates that caspase activation during apoptosis is a rapid process. Role of caspase-3. J. Biol. Chem. 277, 24506–24514 (2002).
Bienko, M. et al. Regulation of translesion synthesis DNA polymerase eta by monoubiquitination. Mol. Cell 37, 396–407 (2010).
Hoeller, D. et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat. Cell Biol. 8, 163–169 (2006).
Terrell, J., Shih, S., Dunn, R. & Hicke, L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol. Cell 1, 193–202 (1998).
Hicke, L., Schubert, H.L. & Hill, C.P. Ubiquitin-binding domains. Nat. Rev. Mol. Cell Biol. 6, 610–621 (2005).
Wiesmann, C. et al. Structural Determinants of MALT1 Protease Activity. J. Mol. Biol. 419, 4–21 (2012).
Yu, J.W., Jeffrey, P.D., Ha, J.Y., Yang, X. & Shi, Y. Crystal structure of the mucosa-associated lymphoid tissue lymphoma translocation 1 (MALT1) paracaspase region. Proc. Natl. Acad. Sci. USA 108, 21004–21009 (2011).
Weih, F., Lira, S.A. & Bravo, R. Overexpression of RelB in transgenic mice does not affect IκB alpha levels: differential regulation of RelA and RelB by the inhibitor protein. Oncogene 12, 445–449 (1996).
Uren, A.G. et al. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol. Cell 6, 961–967 (2000).
Boatright, K.M. & Salvesen, G.S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 15, 725–731 (2003).
Todi, S.V. et al. Ubiquitination directly enhances activity of the deubiquitinating enzyme ataxin-3. EMBO J. 28, 372–382 (2009).
Ngo, V.N. et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature 441, 106–110 (2006).
Neal, J.W. & Clipstone, N.A. Calcineurin mediates the calcium-dependent inhibition of adipocyte differentiation in 3T3–L1 cells. J. Biol. Chem. 277, 49776–49781 (2002).
Annunziata, C.M. et al. Frequent engagement of the classical and alternative NF-κB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12, 115–130 (2007).
Acknowledgements
We thank S. Valitutti (Centre Hospitalier Universitaire Purpan) for monoclonal antibody OKT3; R. Iggo (University of Bordeaux) for lentiviral vector pRDI_292 containing the EF1 promoter; J.-E. Charton (University of Lausanne) for purified T cells; M. Jaworski (University of Lausanne) for the lentiviral NF-κB reporter construct; Z. Chen (University of Texas Southwestern Medical Center) for ubiquitin mutants; N. Clipstone (Loyola University) for the pMSCV-IRES-GFP vector; K. Maslowski for manuscript corrections; S. Hailfinger for support and advice; K. Hofmann and I. Dikic for advice; and F. Martinon for critical reading of the manuscript. Supported by the Swiss National Science Foundation, the Swiss Cancer League (Oncosuisse), Fondation Pierre Mercier Pour la Science, Fondation Emma Muschamp, a collaboration agreement with Ono Pharmaceutical, the Boehringer Ingelheim Foundation (K.C.), the German Research Foundation (G.L.) and the Deutsche Krebshilfe (G.L.).
Author information
Authors and Affiliations
Contributions
C.P. designed and did experiments, analyzed data and wrote the manuscript; K.C. and A.W. contributed to the experiments in Figures 5 and 7d, respectively; M.G. provided technical assistance; G.L. designed and analyzed experiments in Figure 7d and M.T. designed and organized the study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
Part of the work by C.P., K.C., M.G. & M.T. was supported by a collaboration agreement with Ono Pharmaceutical.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 6965 kb)
Rights and permissions
About this article
Cite this article
Pelzer, C., Cabalzar, K., Wolf, A. et al. The protease activity of the paracaspase MALT1 is controlled by monoubiquitination. Nat Immunol 14, 337–345 (2013). https://doi.org/10.1038/ni.2540
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2540
This article is cited by
-
Convergent Molecular Pathways in Type 2 Diabetes Mellitus and Parkinson’s Disease: Insights into Mechanisms and Pathological Consequences
Molecular Neurobiology (2022)
-
A20 and ABIN-1 cooperate in balancing CBM complex-triggered NF-κB signaling in activated T cells
Cellular and Molecular Life Sciences (2022)
-
CARD–BCL-10–MALT1 signalling in protective and pathological immunity
Nature Reviews Immunology (2019)
-
Hectd3 promotes pathogenic Th17 lineage through Stat3 activation and Malt1 signaling in neuroinflammation
Nature Communications (2019)
-
Ways and waves of MALT1 paracaspase activation
Cellular & Molecular Immunology (2018)