Abstract
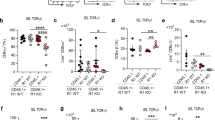
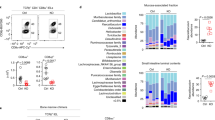
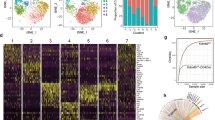
The molecular mechanisms that direct the development of TCRαβ+CD8αα+ intestinal intraepithelial lymphocytes (IELs) are not thoroughly understood. Here we show that transforming growth factor-β (TGF-β) controls the development of TCRαβ+CD8αα+ IELs. Mice with either a null mutation in the gene encoding TGF-β1 or T cell–specific deletion of TGF-β receptor I lacked TCRαβ+CD8αα+ IELs, whereas mice with transgenic overexpression of TGF-β1 had a larger population of TCRαβ+CD8αα+ IELs. We observed defective development of the TCRαβ+CD8αα+ IEL thymic precursors (CD4−CD8−TCRαβ+CD5+) in the absence of TGF-β. In addition, we found that TGF-β signaling induced CD8α expression in TCRαβ+CD8αα+ IEL thymic precursors and induced and maintained CD8α expression in peripheral populations of T cells. Our data demonstrate a previously unrecognized role for TGF-β in the development of TCRαβ+CD8αα+ IELs and the expression of CD8α in T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
van Wijk, F. & Cheroutre, H. Intestinal T cells: facing the mucosal immune dilemma with synergy and diversity. Semin. Immunol. 21, 130–138 (2009).
Denning, T.L. et al. Mouse TCRαβ+CD8αα intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J. Immunol. 178, 4230–4239 (2007).
Saurer, L. et al. Virus-induced activation of self-specific TCRαβ CD8αα intraepithelial lymphocytes does not abolish their self-tolerance in the intestine. J. Immunol. 172, 4176–4183 (2004).
Yamagata, T., Mathis, D. & Benoist, C. Self-reactivity in thymic double-positive cells commits cells to a CD8αα lineage with characteristics of innate immune cells. Nat. Immunol. 5, 597–605 (2004).
Poussier, P., Ning, T., Banerjee, D. & Julius, M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J. Exp. Med. 195, 1491–1497 (2002).
Guy-Grand, D. & Vassalli, P. Immunology. Tracing an orphan's genealogy. Science 305, 185–187 (2004).
Gangadharan, D. et al. Identification of pre- and postselection TCRαβ+ intraepithelial lymphocyte precursors in the thymus. Immunity 25, 631–641 (2006).
Eberl, G. & Littman, D.R. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251 (2004).
Leishman, A.J. et al. Precursors of functional MHC class I- or class II-restricted CD8αα+ T cells are positively selected in the thymus by agonist self-peptides. Immunity 16, 355–364 (2002).
Rocha, B., Vassalli, P. & Guy-Grand, D. The Vβ repertoire of mouse gut homodimeric αCD8+ intraepithelial T cell receptor α/β+ lymphocytes reveals a major extrathymic pathway of T cell differentiation. J. Exp. Med. 173, 483–486 (1991).
Ma, L.J., Acero, L.F., Zal, T. & Schluns, K.S. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8αα IELs. J. Immunol. 183, 1044–1054 (2009).
Apostolou, I., Sarukhan, A., Klein, L. & von Boehmer, H. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3, 756–763 (2002).
Kronenberg, M. & Gapin, L. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2, 557–568 (2002).
Liu, Y. et al. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 9, 632–640 (2008).
Doisne, J.M. et al. iNKT cell development is orchestrated by different branches of TGF-β signaling. J. Exp. Med. 206, 1365–1378 (2009).
Kuo, S., El Guindy, A., Panwala, C.M., Hagan, P.M. & Camerini, V. Differential appearance of T cell subsets in the large and small intestine of neonatal mice. Pediatr. Res. 49, 543–551 (2001).
Podd, B.S., Aberg, C., Christopher, T.L., Perez-Cano, F. & Camerini, V. Late postnatal expansion of self-reactive CD8αα+ intestinal intraepithelial lymphocytes in mice. Autoimmunity 37, 537–547 (2004).
Christ, M. et al. Immune dysregulation in TGF-β 1-deficient mice. J. Immunol. 153, 1936–1946 (1994).
Derynck, R. & Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 (2003).
Hall, B.E. et al. Conditional overexpression of TGF-β 1 disrupts mouse salivary gland development and function. Lab. Invest. 90, 543–555 (2010).
Chen, W. et al. Requirement for transforming growth factor β1 in controlling T cell apoptosis. J. Exp. Med. 194, 439–453 (2001).
Ouyang, W., Beckett, O., Ma, Q. & Li, M.O. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32, 642–653 (2010).
Maraskovsky, E. et al. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell 89, 1011–1019 (1997).
Park, J.H. et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat. Immunol. 11, 257–264 (2010).
Xue, H.H. et al. IL-2 negatively regulates IL-7 receptor α chain expression in activated T lymphocytes. Proc. Natl. Acad. Sci. USA 99, 13759–13764 (2002).
Staton, T.L. et al. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat. Immunol. 7, 482–488 (2006).
Mintern, J.D., Maurice, M.M., Ploegh, H.L. & Schott, E. Thymic selection and peripheral activation of CD8 T cells by the same class I MHC/peptide complex. J. Immunol. 172, 699–708 (2004).
Parker, C.M. et al. A family of β 7 integrins on human mucosal lymphocytes. Proc. Natl. Acad. Sci. USA 89, 1924–1928 (1992).
Wang, L. & Bosselut, R. CD4–CD8 lineage differentiation: Thpok-ing into the nucleus. J. Immunol. 183, 2903–2910 (2009).
Zamisch, M. et al. The transcription factor Ets1 is important for CD4 repression and Runx3 up-regulation during CD8 T cell differentiation in the thymus. J. Exp. Med. 206, 2685–2699 (2009).
Wang, L. et al. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4+ T cells. Nat. Immunol. 9, 1122–1130 (2008).
Li, M.O., Sanjabi, S. & Flavell, R.A. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25, 455–471 (2006).
Parel, Y. & Chizzolini, C. CD4+CD8+ double positive (DP) T cells in health and disease. Autoimmun. Rev. 3, 215–220 (2004).
Das, G. et al. An important regulatory role for CD4+CD8αα T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 100, 5324–5329 (2003).
Wang, L. et al. The zinc finger transcription factor Zbtb7b represses CD8-lineage gene expression in peripheral CD4+ T cells. Immunity 29, 876–887 (2008).
Sun, G. et al. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat. Immunol. 6, 373–381 (2005).
Chen, W. et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Marie, J.C., Letterio, J.J., Gavin, M. & Rudensky, A.Y. TGF-β 1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 201, 1061–1067 (2005).
Lefrancois, L. Phenotypic complexity of intraepithelial lymphocytes of the small intestine. J. Immunol. 147, 1746–1751 (1991).
Hostert, A. et al. A CD8 genomic fragment that directs subset-specific expression of CD8 in transgenic mice. J. Immunol. 158, 4270–4281 (1997).
Blanc, D. et al. Gene transfer of the Ly-3 chain gene of the mouse CD8 molecular complex: co-transfer with the Ly-2 polypeptide gene results in detectable cell surface expression of the Ly-3 antigenic determinants. Eur. J. Immunol. 18, 613–619 (1988).
Sato, T. et al. Dual functions of Runx proteins for reactivating CD8 and silencing CD4 at the commitment process into CD8 thymocytes. Immunity 22, 317–328 (2005).
Hanai, J. et al. Interaction and functional cooperation of PEBP2/CBF with Smads. Synergistic induction of the immunoglobulin germline Cα promoter. J. Biol. Chem. 274, 31577–31582 (1999).
Miyazono, K., Maeda, S. & Imamura, T. Coordinate regulation of cell growth and differentiation by TGF-β superfamily and Runx proteins. Oncogene 23, 4232–4237 (2004).
Klunker, S. et al. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J. Exp. Med. 206, 2701–2715 (2009).
Grueter, B. et al. Runx3 regulates integrin αE/CD103 and CD4 expression during development of CD4−/CD8+ T cells. J. Immunol. 175, 1694–1705 (2005).
Ellmeier, W., Sunshine, M.J., Losos, K., Hatam, F. & Littman, D.R. An enhancer that directs lineage-specific expression of CD8 in positively selected thymocytes and mature T cells. Immunity 7, 537–547 (1997).
Yang, X. et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J. 18, 1280–1291 (1999).
Hall, J.A. et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 29, 637–649 (2008).
Acknowledgements
We thank G. McGrady and S. Wahl (National Institute of Dental and Craniofacial Research, National Institutes of Health) for Tgfb1−/− mice, and E. Stregevsky and J. Simone for technical assistance. Supported by the Intramural Research Program of the National Institutes of Health, National Institute of Dental and Craniofacial Research and National Cancer Institute, Center for Cancer Research.
Author information
Authors and Affiliations
Contributions
J.E.K. designed and did experiments, analyzed data and wrote the manuscript; T.M., B.F.Z. and P.Z. did experiments; B.E.H. and A.B.K. provided critical materials; A.C.C. and Y.X. did experiments and provided critical materials, R.B. provided materials and read the manuscript; and W.C. initiated and directed the research, designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 8275 kb)
Rights and permissions
About this article
Cite this article
Konkel, J., Maruyama, T., Carpenter, A. et al. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat Immunol 12, 312–319 (2011). https://doi.org/10.1038/ni.1997
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1997
This article is cited by
-
TGF-β controls development of TCRγδ+CD8αα+ intestinal intraepithelial lymphocytes
Cell Discovery (2023)
-
The transcription factor LRF promotes integrin β7 expression by and gut homing of CD8αα+ intraepithelial lymphocyte precursors
Nature Immunology (2022)
-
Human mucosal tissue-resident memory T cells in health and disease
Mucosal Immunology (2022)
-
TGFβ control of immune responses in cancer: a holistic immuno-oncology perspective
Nature Reviews Immunology (2022)
-
The histone demethylase Kdm6b regulates the maturation and cytotoxicity of TCRαβ+CD8αα+ intestinal intraepithelial lymphocytes
Cell Death & Differentiation (2022)