Abstract
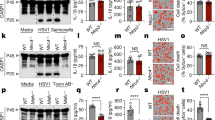
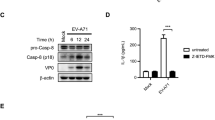
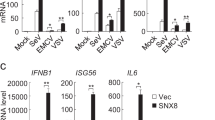
Caspase-12 has been shown to negatively modulate inflammasome signaling during bacterial infection. Its function in viral immunity, however, has not been characterized. We now report an important role for caspase-12 in controlling viral infection via the pattern-recognition receptor RIG-I. After challenge with West Nile virus (WNV), caspase-12-deficient mice had greater mortality, higher viral burden and defective type I interferon response compared with those of challenged wild-type mice. In vitro studies of primary neurons and mouse embryonic fibroblasts showed that caspase-12 positively modulated the production of type I interferon by regulating E3 ubiquitin ligase TRIM25–mediated ubiquitination of RIG-I, a critical signaling event for the type I interferon response to WNV and other important viral pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lamkanfi, M., Festjens, N., Declercq, W., Vanden Berghe, T. & Vandenabeele, P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 14, 44–55 (2007).
Scott, A.M. & Saleh, M. The inflammatory caspases: guardians against infections and sepsis. Cell Death Differ. 14, 23–31 (2007).
Saleh, M. et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429, 75–79 (2004).
Saleh, M. et al. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 440, 1064–1068 (2006).
Franchi, L., Eigenbrod, T., Munoz-Planillo, R. & Nunez, G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10, 241–247 (2009).
Ichinohe, T., Lee, H.K., Ogura, Y., Flavell, R. & Iwasaki, A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206, 79–87 (2009).
Allen, I.C. et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30, 556–565 (2009).
Thomas, P.G. et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30, 566–575 (2009).
Poeck, H. et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nat. Immunol. 11, 63–69 (2009).
Roy, S. et al. Confinement of caspase-12 proteolytic activity to autoprocessing. Proc. Natl. Acad. Sci. USA 105, 4133–4138 (2008).
LeBlanc, P.M. et al. Caspase-12 modulates NOD signaling and regulates antimicrobial peptide production and mucosal immunity. Cell Host Microbe 3, 146–157 (2008).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 (2000).
Tan, Y. et al. Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 281, 16016–16024 (2006).
Yoneda, T. et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 276, 13935–13940 (2001).
Rao, R.V. et al. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J. Biol. Chem. 276, 33869–33874 (2001).
Groenendyk, J. & Michalak, M. Endoplasmic reticulum quality control and apoptosis. Acta Biochim. Pol. 52, 381–395 (2005).
Rao, R.V. et al. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J. Biol. Chem. 277, 21836–21842 (2002).
Obeng, E.A. & Boise, L.H. Caspase-12 and caspase-4 are not required for caspase-dependent endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 280, 29578–29587 (2005).
Di Sano, F. et al. Endoplasmic reticulum stress induces apoptosis by an apoptosome-dependent but caspase 12-independent mechanism. J. Biol. Chem. 281, 2693–2700 (2006).
Martinon, F. & Tschopp, J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 14, 10–22 (2007).
Barton, G.M. & Medzhitov, R. Linking Toll-like receptors to IFN-α/β expression. Nat. Immunol. 4, 432–433 (2003).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Yoneyama, M. et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175, 2851–2858 (2005).
Wang, T. et al. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 10, 1366–1373 (2004).
Daffis, S., Samuel, M.A., Suthar, M.S., Gale, M. Jr & Diamond, M.S. Toll-like receptor 3 has a protective role against West Nile virus infection. J. Virol. 82, 10349–10358 (2008).
Town, T. et al. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity 30, 242–253 (2009).
Fredericksen, B.L., Keller, B.C., Fornek, J., Katze, M.G. & Gale, M. Jr. Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J. Virol. 82, 609–616 (2008).
Loo, Y.M. et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82, 335–345 (2008).
Gack, M.U. et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446, 916–920 (2007).
Arimoto, K. et al. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc. Natl. Acad. Sci. USA 104, 7500–7505 (2007).
Jounai, N. et al. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc. Natl. Acad. Sci. USA 104, 14050–14055 (2007).
Saito, T. et al. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl. Acad. Sci. USA 104, 582–587 (2007).
Satoh, T. et al. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc. Natl. Acad. Sci. USA 107, 1512–1517 (2010).
Simon, D. et al. Friedreich ataxia mouse models with progressive cerebellar and sensory ataxia reveal autophagic neurodegeneration in dorsal root ganglia. J. Neurosci. 24, 1987–1995 (2004).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Yoneyama, M. & Fujita, T. Function of RIG-I-like receptors in antiviral innate immunity. J. Biol. Chem. 282, 15315–15318 (2007).
Fabian, Z., Csatary, C.M., Szeberenyi, J. & Csatary, L.K. p53-independent endoplasmic reticulum stress-mediated cytotoxicity of a Newcastle disease virus strain in tumor cell lines. J. Virol. 81, 2817–2830 (2007).
Li, X.D., Lankinen, H., Putkuri, N., Vapalahti, O. & Vaheri, A. Tula hantavirus triggers pro-apoptotic signals of ER stress in Vero E6 cells. Virology 333, 180–189 (2005).
Jordan, R., Wang, L., Graczyk, T.M., Block, T.M. & Romano, P.R. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J. Virol. 76, 9588–9599 (2002).
Bitko, V. & Barik, S. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J. Cell. Biochem. 80, 441–454 (2001).
Samuel, M.A., Morrey, J.D. & Diamond, M.S. Caspase 3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J. Virol. 81, 2614–2623 (2007).
Shrestha, B., Gottlieb, D. & Diamond, M.S. Infection and injury of neurons by West Nile encephalitis virus. J. Virol. 77, 13203–13213 (2003).
Sejvar, J.J. et al. Neurocognitive and functional outcomes in persons recovering from West Nile virus illness. J. Neuropsychol. 2, 477–499 (2008).
Kobayashi, K. et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416, 194–199 (2002).
Takahashi, K. et al. Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J. Immunol. 176, 4520–4524 (2006).
Kim, M.J. & Yoo, J.Y. Active caspase-1-mediated secretion of retinoic acid inducible gene-I. J. Immunol. 181, 7324–7331 (2008).
Tian, Z., Shen, X., Feng, H. & Gao, B. IL-1 beta attenuates IFN-αβ-induced antiviral activity and STAT1 activation in the liver: involvement of proteasome-dependent pathway. J. Immunol. 165, 3959–3965 (2000).
Yeretssian, G. et al. Gender differences in expression of the human caspase-12 long variant determines susceptibility to Listeria monocytogenes infection. Proc. Natl. Acad. Sci. USA 106, 9016–9020 (2009).
Escribese, M.M. et al. Estrogen inhibits dendritic cell maturation to RNA viruses. Blood 112, 4574–4584 (2008).
Clark, H.B. et al. Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J. Neurosci. 17, 7385–7395 (1997).
Acknowledgements
We thank J.F. Anderson (Connecticut Agricultural Experiment Station) for WNV, and J.U. Jung (University of Southern California) for plasmids. Supported by the National Institutes of Health (AI-055749 and AI-50031), the Howard Hughes Medical Institute (E.F.), the Northeast Biodefense Center (U54-AI057158-Lipkin to P.W.) and the Canadian Institutes for Health Research (79410 to M.S.).
Author information
Authors and Affiliations
Contributions
P.W., A.A., M.S. and E.F. conceived hypotheses, analyzed data and prepared the manuscript; P.W. and A.A. designed and did experiments; Y.Z., H.S., J.D., L.Y., P.M.L. and K.D. aided in experiments; and all authors discussed the results and implications and commented on the manuscript at all stages. E.F. and M.S. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 262 kb)
Supplementary Video 1
Footprint-WT. Movie of wild type mouse on 5 day post i.p. infection with 1500 PFU of WNV (MOV 3617 kb)
Supplementary Video 2
Footprint-Casp12 KO. Movie of Caspase-12 knockout mouse on 5 day post i.p. infection with 1500 PFU of WNV (MOV 4826 kb)
Supplementary Video 3
Footprint-Casp1 KO. Movie of Caspase-12 knockout mouse on 5 day post i.p. infection with 1500 PFU of WNV (MOV 7610 kb)
Rights and permissions
About this article
Cite this article
Wang, P., Arjona, A., Zhang, Y. et al. Caspase-12 controls West Nile virus infection via the viral RNA receptor RIG-I. Nat Immunol 11, 912–919 (2010). https://doi.org/10.1038/ni.1933
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1933
This article is cited by
-
Regulation of RIG-I-like receptor-mediated signaling: interaction between host and viral factors
Cellular & Molecular Immunology (2021)
-
RIG-I-like receptors: their regulation and roles in RNA sensing
Nature Reviews Immunology (2020)
-
Macrophage scavenger receptor 1 controls Chikungunya virus infection through autophagy in mice
Communications Biology (2020)
-
Intracellular sensing of viral genomes and viral evasion
Experimental & Molecular Medicine (2019)
-
UBXN3B positively regulates STING-mediated antiviral immune responses
Nature Communications (2018)