Abstract
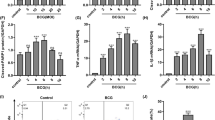
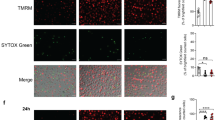
Apoptosis is central to the interaction between pathogenic mycobacteria and host macrophages. Caspase-8-dependent apoptosis of infected macrophages, which requires activation of the mitogen-activated protein (MAP) kinase p38, lowers the spread of mycobacteria. Here we establish a link between the release of tumor necrosis factor (TNF) and mycobacteria-mediated macrophage apoptosis. TNF activated a pathway involving the kinases ASK1, p38 and c-Abl. This pathway led to phosphorylation of FLIPS, which facilitated its interaction with the E3 ubiquitin ligase c-Cbl. This interaction triggered proteasomal degradation of FLIPS, which promoted activation of caspase-8 and apoptosis. Our findings identify a previously unappreciated signaling pathway needed for Mycobacterium tuberculosis–triggered macrophage cell death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Aggarwal, B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745–756 (2003).
Boldin, M.P., Goncharov, T.M., Goltsev, Y.V. & Wallach, D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1-and TNF receptor-induced cell death. Cell 85, 803–815 (1996).
Chinnaiyan, A.M., O'Rourke, K., Tewari, M. & Dixit, V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81, 505–512 (1995).
Muppidi, J.R., Tschopp, J. & Siegel, R.M. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity 21, 461–465 (2004).
Hsu, H., Huang, J., Shu, H.B., Baichwal, V. & Goeddel, D.V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 (1996).
Rothe, M., Wong, S.C., Henzel, W.J. & Goeddel, D.V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell 78, 681–692 (1994).
Chen, C., Edelstein, L.C. & Gelinas, C. The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-xL . Mol. Cell. Biol. 20, 2687–2695 (2000).
Chu, Z.L. et al. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc. Natl. Acad. Sci. USA 94, 10057–10062 (1997).
Goltsev, Y.V. et al. CASH, a novel caspase homologue with death effector domains. J. Biol. Chem. 272, 19641–19644 (1997).
Hu, S., Vincenz, C., Ni, J., Gentz, R. & Dixit, V.M. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95-induced apoptosis. J. Biol. Chem. 272, 17255–17257 (1997).
Irmler, M. et al. Inhibition of death receptor signals by cellular FLIP. Nature 388, 190–195 (1997).
Shu, H.B., Halpin, D.R. & Goeddel, D.V. Casper is a FADD- and caspase-related inducer of apoptosis. Immunity 6, 751–763 (1997).
Gloks, A., Brenner, D., Fritsch, C., Krammer, P.H. & Lavrik, I.N. c-FLIPR, a new regulator of death receptor-induced apoptosis. J. Biol. Chem. 280, 14507–14513 (2005).
Krueger, A., Baumann, S., Krammer, P.H. & Kirchoff, S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol. Cell. Biol. 21, 8247–8254 (2001).
Kataoka, T. et al. The caspase-8 inhibitor FLIP promotes activation of NF-κB and ERK signaling pathways. Curr. Biol. 10, 640–648 (2000).
Perez, D. & White, E. E1A sensitizes cells to tumor necrosis factor α by downregulating c-FLIPS . J. Virol. 77, 2651–2662 (2003).
Schaible, U.E. et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 9, 1039–1046 (2003).
Ichijo, H. et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275, 90–94 (1997).
Bhattacharyya, A. et al. Execution of macrophage apoptosis by Mycobacterium avium through apoptosis signal-regulating kinase 1/p38 mitogen-activated protein kinase signaling and caspase 8 activation. J. Biol. Chem. 278, 26517–26525 (2003).
Noguchi, T. et al. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J. Biol. Chem. 283, 7657–7665 (2008).
Karin, M. & Lin, A.N.F. -κB at the crossroads of life and death. Nat. Immunol. 3, 221–227 (2002).
Grethea, S., Aresb, M.P.S., Anderssona, T. & Pörn-Ares, M.I. p38 MAPK mediates TNF-induced apoptosis in endothelial cells via phosphorylation and downregulation of Bcl-xL. Exp. Cell Res. 298, 632–642 (2004).
Perlman, H. et al. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to Fas-mediated apoptosis. J. Exp. Med. 190, 1679–1688 (1999).
Kim, Y., Shuh, N., Sporn, M. & Reed, J.C. An inducible pathway for degradation of FLIP protein sensitizes cells to TRAIL-induced apoptosis. J. Biol. Chem. 277, 22320–22329 (2002).
Tourian, L., Jr, Zhao, H. & Srikant, C.B. p38α, but not p38β, inhibits the phosphorylation and presence of c-FLIPS in Drosoph. Inf. Serv.C to potentiate Fas-mediated caspase-8 activation and type I apoptotic signaling. J. Cell Sci. 117, 6459–6471 (2004).
Holland, P.M. & Cooper, J.A. Protein modification: Docking sites for kinases. Curr. Biol. 9, R329–R331 (1999).
Poukkula, M. et al. Rapid turnover of c-FLIPshort is determined by its unique C-terminal tail. J. Biol. Chem. 280, 27345–27355 (2005).
Abella, J.V. et al. Met/Hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for Hrs phosphorylation. Mol. Cell. Biol. 25, 9632–9645 (2005).
Le, Q. et al. c-Abl tyrosine kinase is also involved in LPS-mediated activation of macrophages: reduction of c-Abl leads to inhibition of macrophage activation induced by LPS and induction of apoptosis in macrophages treated by LPS. J. Immunol. 160, 3330–3336 (1998).
Levkowitz, G. et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell 4, 1029–1040 (1999).
Thien, C.B. & Langdon, W.Y. Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell Biol. 2, 294–307 (2001).
Schmidt, M.H. & Dikic, I. The Cbl interactome and its functions. Nat. Rev. Mol. Cell Biol. 6, 907–919 (2005).
Oksvold, M.P. et al. Serine mutations that abrogate ligand-induced ubiquitination and internalization of the EGF receptor do not affect c-Cbl association with the receptor. Oncogene 22, 8509–8518 (2003).
Kyo, S. et al. Negative regulation of Lyn protein-tyrosine kinase by c-Cbl ubiquitin-protein ligase in Fc epsilon RI-mediated mast cell activation. Genes Cells 8, 825–836 (2003).
Xiong, H. et al. Ubiquitin-dependent degradation of interferon regulatory factor-8 mediated by Cbl down-regulates interleukin-12 expression. J. Biol. Chem. 280, 23531–23539 (2005).
Schaible, U. et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 9, 1039–1045 (2003).
Chen, M., Gan, H. & Remold, G.G. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J. Immunol. 176, 3703–3716 (2006).
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 25, 181–190 (2003).
Rhoades, E.R., Cooper, A.M. & Orme, I.M. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect. Immun. 63, 3871–3877 (1995).
Flynn, J.L. et al. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2, 561–572 (1995).
Park, J.M., Greten, F.R., Li, Z.W. & Karin, M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297, 2048–2051 (2002).
Backert, S., Feller, S.M. & Wessler, S. Emerging roles of Abl family tyrosine kinases in microbial pathogenesis. Trends Biochem. Sci. 33, 80–90 (2007).
Jesenberger, V. & Jentsch, S. Deadly encounter: ubiquitin meets apoptosis. Nat. Rev. Mol. Cell Biol. 3, 112–121 (2002).
Chang, L. et al. The E3 ubiquitin ligase Itch couples JNK activation to TNFα-induced cell death by inducing c-FLIPL turnover. Cell 124, 601–613 (2006).
Yokouchi, M. et al. Src-catalyzed phosphorylation of c-Cbl leads to the interdependent ubiquitination of both proteins. J. Biol. Chem. 276, 35185–35193 (2001).
Echarri, A. & Pendergast, A.M. Activated c-Abl is degraded by the ubiquitin-dependent proteasome pathway. Curr. Biol. 11, 1759–1765 (2001).
Gómez-Muñoz, A., Kong, J.Y., Salh, B. & Steinbrecher, U.P. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J. Lipid Res. 45, 99–105 (2004).
Murphy, M.A. et al. Hyperplasia and enhanced T cell signalling via ZAP-70 in c-Cbl deficient mice. Mol. Cell. Biol. 18, 4872–4882 (1998).
Tobiume, K. et al. ASK1 is required for sustained activation of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2, 222–228 (2001).
Pathak, S.K. et al. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat. Immunol. 8, 610–618 (2007).
Acknowledgements
We thank R. Davis (University of Massachusetts Medical School) for Flag-tagged p38 and p38(dn) and for Jnk and its dominant negative mutant; J. Han (Scripps Research Institute) for bacterial MKK6(E) and p38 constructs; and G. Superti-Furga (Austrian Academy of Science) for c-Abl and kinase-inactive c-Abl in pSGT. Supported by the Council of Scientific and Industrial Research of the Government of India (S.K.P.) and the Department of Science and Technology of the Government of India (J.B.).
Author information
Authors and Affiliations
Contributions
M.K. designed and did research, analyzed data and wrote the paper; S.K.P., K.K., S.B., G.C. and S.P. did research; T.N., K.T., H.I., C.B.F.T. and W.Y.L. contributed tools; and J.B. designed the research, analyzed data and wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 758 kb)
Rights and permissions
About this article
Cite this article
Kundu, M., Pathak, S., Kumawat, K. et al. A TNF- and c-Cbl-dependent FLIPS-degradation pathway and its function in Mycobacterium tuberculosis–induced macrophage apoptosis. Nat Immunol 10, 918–926 (2009). https://doi.org/10.1038/ni.1754
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1754
This article is cited by
-
Early secreted antigenic target of 6-kDa of Mycobacterium tuberculosis promotes caspase-9/caspase-3-mediated apoptosis in macrophages
Molecular and Cellular Biochemistry (2019)
-
Rv2346c enhances mycobacterial survival within macrophages by inhibiting TNF-α and IL-6 production via the p38/miRNA/NF-κB pathway
Emerging Microbes & Infections (2018)
-
Multidrug-resistant tuberculosis (MDR-TB) strain infection in macaques results in high bacilli burdens in airways, driving broad innate/adaptive immune responses
Emerging Microbes & Infections (2018)
-
Hyperthermia restores apoptosis induced by death receptors through aggregation-induced c-FLIP cytosolic depletion
Cell Death & Disease (2015)
-
Bim is a crucial regulator of apoptosis induced by Mycobacterium tuberculosis
Cell Death & Disease (2014)