Abstract
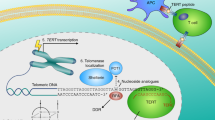
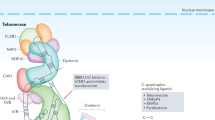
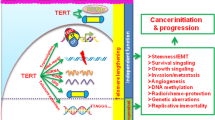
Inhibition of telomerase is proposed to limit the growth of cancer cells by triggering telomere shortening and cell death1,2,3,4,5,6,7,8,9. Telomere maintenance by telomerase is sufficient, in some cell types, to allow immortal growth1,2,3,4,5. Telomerase has been shown to cooperate with oncogenes in transforming cultured primary human cells into neoplastic cells, suggesting that telomerase activation contributes to malignant transformation6. Moreover, telomerase inhibition in human tumour cell lines using dominant-negative versions of TERT leads to telomere shortening and cell death7,8. These findings have led to the proposition that telomerase inhibition may result in cessation of tumour growth9. The absence of telomerase from most normal cells supports the potential efficacy of anti-telomerase drugs for tumour therapy, as its inhibition is unlikely to have toxic effects. Mice deficient for Terc RNA (encoding telomerase) lack telomerase activity, and constitute a model for evaluating the role of telomerase and telomeres in tumourigenesis10. Late-generation Terc−/− mice show defects in proliferative tissues10,11,12,13,14,15 and a moderate increase in the incidence of spontaneous tumours in highly proliferative cell types (lymphomas, teratocarcinomas12). The appearance of these tumours is thought to be a consequence of chromosomal instability in these mice10,12,16,17. These observations have challenged the expected effectiveness of anti-telomerase–based cancer therapies18. Different cell types may nonetheless vary in their sensitivity to the chromosomal instability produced by telomere loss or to the activation of telomere-rescue mechanisms. Here we show that late-generation Terc−/− mice, which have short telomeres and are telomerase-deficient10, are resistant to tumour development in multi-stage skin carcinogenesis. Our results predict that an anti-telomerase–based tumour therapy may be effective in epithelial tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bodnar, A.G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Wang, J., Xie, L.Y., Allan, S., Beach, D. & Hannon, G.J. Myc activates telomerase. Genes Dev. 12, 1769–1774 (1998).
Kiyono, T. et al. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396, 84–88 (1998).
Morales, C.P. et al. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nature Genet. 21, 115–118 (1999).
Jiang, X.-R. et al. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nature Genet. 21, 111–114 (1999).
Hahn, W.C. et al. Creation of human tumour cells with defined genetic elements. Nature 400, 464–468 (1999).
Hahn, W.C. et al. Inhibition of telomerase limits the growth of human cancer cells. Nature Med. 5, 1164–1170 (1999).
Zhang, X., Mar, V., Zhou, W., Harrington, L. & Robinson, M.O. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 13, 2388–2399 (1999).
Zumstein, L.A. & Lundblad, V. Telomeres: has cancer's Achilles' heel been exposed? Nature Med. 5, 1129–1130 (1999).
Blasco, M.A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Lee, H.-W. et al. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998).
Rudolph, K.L. et al. Longevity, stress response, and cancer in aging telomerase deficient mice. Cell 96, 701–712 (1999).
Herrera, E., Samper, E. & Blasco, M.A. Telomere shortening in Terc−/− embryos is associated with failure to close the neural tube. EMBO J. 18, 1172–1181 (1999).
Herrera, E. et al. Disease states associated to telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 18, 2950–2960 (1999).
Herrera, E., Martínez- A., C. & Blasco, M.A. Impaired germinal center formation in telomerase-deficient mice. EMBO J. 19, 472–481 (2000).
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere sysfunction to accelerate carcinogenesis. Cell 97, 527–538 (1999).
Greenberg, R.A. et al. Short dysfunctional telomeres impair tumorigenesis in the INK4aΔ2/3 cancer-prone mouse. Cell 97, 515–525 (1999).
De Lange, T. & Jacks, T. For better or worse? Telomerase inhibition and cancer. Cell 98, 273–275 (1999).
Heckner, E., Fusenig, N.E., Kunz, W., Marks, F. & Thielmann, H.W. (eds) Carcinogenesis: A Comprehensive Survey (Raven, New York, 1982).
Balmain, A. et al. in Multistage Carcinogenesis (eds Harris, C.C. et al.) 97–108 (Japan Scientific Society Press/CRC Press, Boca Raton, 1992).
Balmain, A., Ramsden, M., Bowden, G.T. & Smith, J. Activation of the mouse Harvey-ras gene in chemically induced benign skin papillomas. Nature 307, 658–660 (1984).
Quintanilla, M., Brown, K., Ramsden, M. & Balmain, A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature 322, 78–80 (1986).
Bednarek, A.K., Chu, Y., Slaga, I.J. & Aldaz, C.M. Telomerase and cell proliferation in mouse skin papillomas. Mol. Carcinog. 20, 329–310 (1997).
Bryan, T.M., Englezou, A., Gupta, J., Bacchetti, S. & Reddel, R.R. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 14, 4240–4248 (1995).
Bryan, T.M., Marusic, L., Bacchetti, S., Namba, M. & Reddel, R.R. The telomere lengthening mechanism in telomerase-negative immortal human cells does not involve the telomerase RNA subunit. Hum. Mol. Genet. 6, 921–926 (1997).
Zijlmans, J.M. et al. Telomeres in the mouse have large inter-chromosomal variations in the number of T2AG3 repeats. Proc. Natl Acad. Sci. USA 94, 7423–7428 (1997).
Hennings, H. Primary culture of keratinocytes from newborn mouse epidermis in medium with lowered levels of Ca2+. in Keratinocyte Methods (eds Leigh, I.M. & Watt, F.M.) 21–23 (Cambridge University Press, New York, 1994).
Rufer, N., Dragowska, W., Thornbury, G., Roosnek, E. & Lansdorp, P.M. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nature Biotechnol. 16, 743–147 (1998).
Martín-Rivera, L., Herrera, E., Albar, J.P. & Blasco, M.A. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc. Natl Acad. Sci. USA 95, 10471–10476 (1998).
Pantoja, C. & Serrano, M. Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene 18, 4974–4982 (1999).
Acknowledgements
We thank E. Santos and J. Martín-Caballero for mouse care and genotyping, and C. Mark and M. Serrano for critical reading of the manuscript. E.G.-S. is a predoctoral fellow from Fondo de Investigaciones Sanitarias (FIS). E.S. is a predoctoral fellow from Regional Government of Madrid (CAM). Research at the laboratory of M.A.B. is funded by grants PM97-0133 from the Ministry of Science and Technology, Spain, 08.1/0030/98 from CAM, and by grants EURATOM/991/0201, FIGH-CT-1999-00002 and FIS5-1999-00055 from the European Union, and by the DIO. The DIO was founded and is supported by the Spanish Research Council (CSIC) and by The Pharmacia Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Suárez, E., Samper, E., Flores, J. et al. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat Genet 26, 114–117 (2000). https://doi.org/10.1038/79089
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/79089
This article is cited by
-
Regulation of telomerase towards tumor therapy
Cell & Bioscience (2023)
-
Analysis and validation of aging-related genes in prognosis and immune function of glioblastoma
BMC Medical Genomics (2023)
-
Short and dysfunctional telomeres sensitize the kidneys to develop fibrosis
Nature Aging (2021)
-
Non-canonical roles of canonical telomere binding proteins in cancers
Cellular and Molecular Life Sciences (2021)
-
TERT promoter alterations could provide a solution for Peto’s paradox in rodents
Scientific Reports (2020)