Abstract
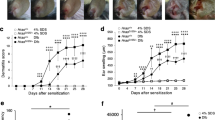
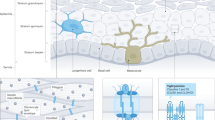
Loss-of-function mutations in the FLG (filaggrin) gene cause the semidominant keratinizing disorder ichthyosis vulgaris1 and convey major genetic risk for atopic dermatitis (eczema)2,3,4, eczema-associated asthma2,3 and other allergic phenotypes5. Several low-frequency FLG null alleles occur in Europeans and Asians, with a cumulative frequency of ∼9% in Europe4. Here we report a 1-bp deletion mutation, 5303delA, analogous to common human FLG mutations, within the murine Flg gene in the spontaneous mouse mutant flaky tail (ft). We demonstrate that topical application of allergen to mice homozygous for this mutation results in cutaneous inflammatory infiltrates and enhanced cutaneous allergen priming with development of allergen-specific antibody responses. These data validate flaky tail as a useful model of filaggrin deficiency and provide experimental evidence for the hypothesis that antigen transfer through a defective epidermal barrier is a key mechanism underlying elevated IgE sensitization and initiation of cutaneous inflammation in humans with filaggrin-related atopic disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Smith, F.J.D. et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat. Genet. 38, 337–342 (2006).
Palmer, C.N.A. et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 38, 441–446 (2006).
Baurecht, H. et al. Toward a major risk factor for atopic eczema: meta-analysis of filaggrin polymorphism data. J. Allergy Clin. Immunol. 120, 1406–1412 (2007).
Sandilands, A. et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat. Genet. 39, 650–654 (2007).
Henderson, J. et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J. Allergy Clin. Immunol. 121, 872–877 (2008).
Williams, H. & Flohr, C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J. Allergy Clin. Immunol. 118, 209–213 (2006).
Spergel, J.M. & Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 112, S118–S127 (2003).
Illi, S. et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J. Allergy Clin. Immunol. 113, 925–931 (2004).
Bieber, T. Atopic dermatitis. N. Engl. J. Med. 358, 1483–1494 (2008).
Sundberg, J.P. in Handbook of Mouse Mutations with Skin and Hair Abnormalities. Animal Models and Biochemical Tools. Vol. 2 (ed. Sundberg, J.P.) The flaky tail (ft) mutation, 269–273 (CRC Press, Ann Arbor, Michigan, USA, 1984).
Presland, R.B. et al. Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris. J. Invest. Dermatol. 115, 1072–1081 (2000).
Pearton, D.J., Dale, B.A. & Presland, R.B. Functional analysis of the profilaggrin N-terminal peptide: identification of domains that regulate nuclear and cytoplasmic distribution. J. Invest. Dermatol. 119, 661–669 (2002).
Rothnagel, J.A., Mehrel, T., Idler, W.W., Roop, D.R. & Steinert, P.M. The gene for mouse epidermal filaggrin precursor. Its partial characterization, expression, and sequence of a repeating filaggrin unit. J. Biol. Chem. 262, 15643–15648 (1987).
Presland, R.B., Bassuk, J.A., Kimball, J.R. & Dale, B.A. Characterization of two distinct calcium-binding sites in the amino-terminus of human profilaggrin. J. Invest. Dermatol. 104, 218–223 (1995).
Zhang, D., Karunaratne, S., Kessler, M., Mahony, D. & Rothnagel, J.A. Characterization of mouse profilaggrin: evidence for nuclear engulfment and translocation of the profilaggrin B-domain during epidermal differentiation. J. Invest. Dermatol. 119, 905–912 (2002).
Rothnagel, J.A. & Steinert, P.M. The structure of the gene for mouse filaggrin and a comparison of the repeating units. J. Biol. Chem. 265, 1862–1865 (1990).
Gan, S.Q., McBride, O.W., Idler, W.W., Markova, N. & Steinert, P.M. Organization, structure, and polymorphisms of the human profilaggrin gene. Biochemistry 29, 9432–9440 (1990).
Sasaki, T. et al. Sequence analysis of filaggrin gene by novel shotgun method in Japanese atopic dermatitis. J. Dermatol. Sci. 51, 113–120 (2008).
Terada, M. et al. Contribution of IL-18 to atopic-dermatitis-like skin inflammation induced by Staphylococcus aureus product in mice. Proc. Natl. Acad. Sci. USA 103, 8816–8821 (2006).
Spergel, J.M., Mizoguchi, E., Oettgen, H., Bhan, A.K. & Geha, R.S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J. Clin. Invest. 103, 1103–1111 (1999).
Snapper, C.M. & Paul, W.E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236, 944–947 (1987).
Weidinger, S. et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J. Allergy Clin. Immunol. 121, 1203–1209 (2008).
Marenholz, I. et al. Filaggrin loss-of-function mutations predispose to phenotypes involved in the atopic march. J. Allergy Clin. Immunol. 118, 866–871 (2006).
McLean, W.H.I. et al. Filaggrin variants confer susceptibility to asthma. J. Allergy Clin. Immunol. 121, 1294–1295 (2008).
Spergel, J.M. et al. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J. Clin. Invest. 101, 1614–1622 (1998).
Howell, M.D. et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 120, 150–155 (2007).
Kim, B.E., Leung, D.Y., Boguniewicz, M. & Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 126, 332–337 (2008).
Matsuda, H. et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 9, 461–466 (1997).
Chen, L., Overbergh, L., Mathieu, C. & Chan, L.S. The development of atopic dermatitis is independent of Immunoglobulin E up-regulation in the K14-IL-4 SKH1 transgenic mouse model. Clin. Exp. Allergy 38, 1367–1380 (2008).
Mangan, N.E., van Rooijen, N., McKenzie, A.N. & Fallon, P.G. Helminth-modified pulmonary immune response protects mice from allergen-induced airway hyperresponsiveness. J. Immunol. 176, 138–147 (2006).
Acknowledgements
We thank B. Mistry, A. Smyth, T. Adachi and M. Furuhashi for their technical assistance and T. Yoshida for his cooperation. We are grateful for the assistance of B. Cloak with photomicrography and S. Worrell with histopathology. This work was supported by the Labour Sciences Research Grants for Research on Allergic Disease and Immunology from the Ministry of Health, Labour, and Welfare of Japan (M.A.), National Institute of Health grants P01 AM21557 (P.F.), R01 AR49183 (R.B.P.) and the Odland Endowed Research Fund (P.F.). P.G.F. was supported by Science Foundation Ireland. A.D.I. is supported by the Children's Medical and Research Foundation, OLCHC. Filaggrin research in the McLean laboratory is supported by grants from The British Skin Foundation, The National Eczema Society, The Medical Research Council (Reference number G0700314) and donations from anonymous families affected by eczema in the Tayside Region of Scotland.
Author information
Authors and Affiliations
Contributions
The study was designed by W.H.I.M., P.G.F., A.D.I and M.A. Molecular biology was performed by T.S., A. Sandilands, L.E.C., H.K., A. Shiohama, A.K., N.S. and J.K. Bioinformatics was performed by W.H.I.M. C-terminal profilaggrin antibody and immunoblot data was generated by R.B.P. and P.F. The initial mixed strain ft mice were provided by J.P.S. Mouse backcrossing, immunology and histological experiments were performed by P.G.F., S.P.S., N.E.M and J.J.C. The manuscript was written by P.G.F., T.S., J.K., M.A., R.B.P., A.D.I. and W.H.I.M.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–3 (PDF 560 kb)
Rights and permissions
About this article
Cite this article
Fallon, P., Sasaki, T., Sandilands, A. et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet 41, 602–608 (2009). https://doi.org/10.1038/ng.358
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.358
This article is cited by
-
Suprabasin-null mice retain skin barrier function and show high contact hypersensitivity to nickel upon oral nickel loading
Scientific Reports (2020)
-
Selective JAK1 Inhibitors for the Treatment of Atopic Dermatitis: Focus on Upadacitinib and Abrocitinib
American Journal of Clinical Dermatology (2020)
-
Topical administration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/Nga mice
Scientific Reports (2018)
-
Atopic dermatitis
Nature Reviews Disease Primers (2018)
-
Pathophysiology of Eosinophilic Esophagitis
Clinical Reviews in Allergy & Immunology (2018)