Abstract
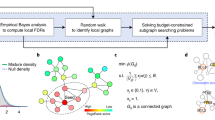
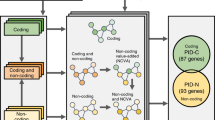
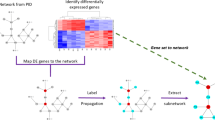
Cancers exhibit extensive mutational heterogeneity, and the resulting long-tail phenomenon complicates the discovery of genes and pathways that are significantly mutated in cancer. We perform a pan-cancer analysis of mutated networks in 3,281 samples from 12 cancer types from The Cancer Genome Atlas (TCGA) using HotNet2, a new algorithm to find mutated subnetworks that overcomes the limitations of existing single-gene, pathway and network approaches. We identify 16 significantly mutated subnetworks that comprise well-known cancer signaling pathways as well as subnetworks with less characterized roles in cancer, including cohesin, condensin and others. Many of these subnetworks exhibit co-occurring mutations across samples. These subnetworks contain dozens of genes with rare somatic mutations across multiple cancers; many of these genes have additional evidence supporting a role in cancer. By illuminating these rare combinations of mutations, pan-cancer network analyses provide a roadmap to investigate new diagnostic and therapeutic opportunities across cancer types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Cancer Genome Atlas Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 (2008).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 99, 43–49 (2013).
Cancer Genome Atlas Network. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012).
Kandoth, C. et al. Integrated genomic characterization of endometrial carcinoma. Nature 497, 67–73 (2013).
Cancer Genome Atlas Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074 (2013).
Stratton, M.R., Campbell, P.J. & Futreal, P.A. The cancer genome. Nature 458, 719–724 (2009).
Vogelstein, B. et al. Cancer genome landscapes. Science 339, 1546–1558 (2013).
Garraway, L.A. & Lander, E.S. Lessons from the cancer genome. Cell 153, 17–37 (2013).
Lawrence, M.S. et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Zack, T.I. et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 45, 1134–1140 (2013).
Weinstein, J.N. et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 45, 1113–1120 (2013).
Hanahan, D. & Weinberg, R.a. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Vandin, F., Upfal, E. & Raphael, B.J. Algorithms for detecting significantly mutated pathways in cancer. J. Comput. Biol. 18, 507–522 (2011).
Vandin, F., Clay, P., Upfal, E. & Raphael, B.J. Discovery of mutated subnetworks associated with clinical data in cancer. Pac. Symp. Biocomput. 2012, 55–66 (2012).
Grasso, C.S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012).
Hofree, M., Shen, J.P., Carter, H., Gross, A. & Ideker, T. Network-based stratification of tumor mutations. Nat. Methods 10, 1108–1115 (2013).
Lawrence, M.S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Das, J. & Yu, H. HINT: high-quality protein interactomes and their applications in understanding human disease. BMC Syst. Biol. 6, 92 (2012).
Yu, H. et al. Next-generation sequencing to generate interactome datasets. Nat. Methods 8, 478–480 (2011).
Khurana, E., Fu, Y., Chen, J. & Gerstein, M. Interpretation of genomic variants using a unified biological network approach. PLOS Comput. Biol. 9, e1002886 (2013).
Razick, S., Magklaras, G. & Donaldson, I.M. iRefIndex: a consolidated protein interaction database with provenance. BMC Bioinformatics 9, 405 (2008).
Hoadley, K.A. et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 158, 929–944 (2014).
Gonzalez-Perez, A. & Lopez-Bigas, N. Functional impact bias reveals cancer drivers. Nucleic Acids Res. 40, e169 (2012).
Tamborero, D., Lopez-Bigas, N. & Gonzalez-Perez, A. Oncodrive-CIS: a method to reveal likely driver genes based on the impact of their copy number changes on expression. PLoS ONE 8, e55489 (2013).
Dees, N.D. et al. MuSiC: identifying mutational significance in cancer genomes. Genome Res. 22, 1589–1598 (2012).
Mermel, C.H. et al. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 12, R41 (2011).
Ye, J., Pavlicek, A., Lunney, E.A., Rejto, P.A. & Teng, C.-H. Statistical method on nonrandom clustering with application to somatic mutations in cancer. BMC Bioinformatics 11, 11 (2010).
Ryslik, G.A., Cheng, Y., Cheung, K.-H., Modis, Y. & Zhao, H. Utilizing protein structure to identify non-random somatic mutations. BMC Bioinformatics 14, 190 (2013).
Yeang, C.-H., McCormick, F. & Levine, A. Combinatorial patterns of somatic gene mutations in cancer. FASEB J. 22, 2605–2622 (2008).
Vandin, F., Upfal, E. & Raphael, B.J. De novo discovery of mutated driver pathways in cancer. Genome Res. 22, 375–385 (2012).
Solis, L.M. et al. Nrf2 and Keap1 abnormalities in non–small cell lung carcinoma and association with clinicopathologic features. Clin. Cancer Res. 16, 3743–3753 (2010).
Yamadori, T. et al. Molecular mechanisms for the regulation of Nrf2-mediated cell proliferation in non-small-cell lung cancers. Oncogene 31, 4768–4777 (2012).
Thompson, B.A., Tremblay, V., Lin, G. & Bochar, D.A. CHD8 is an ATP-dependent chromatin remodeling factor that regulates β-catenin target genes. Mol. Cell. Biol. 28, 3894–3904 (2008).
Greife, A. et al. Canonical Notch signalling is inactive in urothelial carcinoma. BMC Cancer 14, 628 (2014).
Wilson, B.G. & Roberts, C.W.M. SWI/SNF nucleosome remodellers and cancer. Nat. Rev. Cancer 11, 481–492 (2011).
Varela, I. et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469, 539–542 (2011).
Kadoch, C. et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 45, 592–601 (2013).
Sausen, M. et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat. Genet. 45, 12–17 (2013).
Tsurusaki, Y. et al. Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat. Genet. 44, 376–378 (2012).
Mandel, S. & Gozes, I. Activity-dependent neuroprotective protein constitutes a novel element in the SWI/SNF chromatin remodeling complex. J. Biol. Chem. 282, 34448–34456 (2007).
Steingart, R.A. & Gozes, I. Recombinant activity-dependent neuroprotective protein protects cells against oxidative stress. Mol. Cell. Endocrinol. 252, 148–153 (2006).
Carbone, M. et al. BAP1 and cancer. Nat. Rev. Cancer 13, 153–159 (2013).
Peña-Llopis, S. et al. BAP1 loss defines a new class of renal cell carcinoma. Nat. Genet. 44, 751–759 (2012).
Fang, R. et al. Human LSD2/KDM1b/AOF1 regulates gene transcription by modulating intragenic H3K4me2 methylation. Mol. Cell 39, 222–233 (2010).
Shi, Y. et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953 (2004).
Xu, H., Tomaszewski, J.M. & McKay, M.J. Can corruption of chromosome cohesion create a conduit to cancer? Nat. Rev. Cancer 11, 199–210 (2011).
Rubio, E.D. et al. CTCF physically links cohesin to chromatin. Proc. Natl. Acad. Sci. USA 105, 8309–8314 (2008).
Schmidt, D. et al. A CTCF-independent role for cohesin in tissue-specific transcription. Genome Res. 20, 578–588 (2010).
Kon, A. et al. Recurrent mutations in multiple components of the cohesin complex in myeloid neoplasms. Nat. Genet. 45, 1232–1237 (2013).
Solomon, D.A. et al. Frequent truncating mutations of STAG2 in bladder cancer. Nat. Genet. 45, 1428–1430 (2013).
Wood, A.J., Severson, A.F. & Meyer, B.J. Condensin and cohesin complexity: the expanding repertoire of functions. Nat. Rev. Genet. 11, 391–404 (2010).
Hirano, T. Condensins: universal organizers of chromosomes with diverse functions. Genes Dev. 26, 1659–1678 (2012).
Lapointe, J. et al. hCAP-D3 expression marks a prostate cancer subtype with favorable clinical behavior and androgen signaling signature. Am. J. Surg. Pathol. 32, 205–209 (2008).
Ciriello, G. et al. Emerging landscape of oncogenic signatures across human cancers. Nat. Genet. 45, 1127–1133 (2013).
Mitra, K., Carvunis, A.-R., Ramesh, S.K. & Ideker, T. Integrative approaches for finding modular structure in biological networks. Nat. Rev. Genet. 14, 719–732 (2013).
Chung, F. The heat kernel as the pagerank of a graph. Proc. Natl. Acad. Sci. USA 104, 19735–19740 (2007).
Berkhin, P. Bookmark-Coloring algorithm for personalized PageRank computing. Internet Math. 3, 41–62 (2006).
Huang, W., Sherman, B.T. & Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 4, 44–57 (2009).
Huang, W., Sherman, B.T. & Lempicki, R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009).
Mootha, V.K. et al. PGC-1α–responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Ciriello, G., Cerami, E.G., Sander, C. & Schultz, N. Mutual exclusivity analysis identifies oncogenic network modules. Genome Res. 22, 398–406 (2012).
Shay, J.W., Zou, Y., Hiyama, E. & Wright, W.E. Telomerase and cancer. Hum. Mol. Genet. 10, 677–685 (2001).
Acknowledgements
The authors thank F. Roth for his assistance in constructing the HINT+HI2012 interaction network. We gratefully acknowledge the contributions of the TCGA Research Network and its TCGA Pan-Cancer Analysis Working Group. This work is supported by US National Science Foundation (NSF) grant IIS-1016648 and US National Institutes of Health (NIH) grants R01HG005690, R01HG007069 and R01CA180776 to B.J.R. and by National Human Genome Research Institute (NHGRI) grant U01HG006517 to L.D. B.J.R. is supported by a Career Award at the Scientific Interface from the Burroughs Wellcome Fund, an Alfred P. Sloan Research Fellowship and an NSF CAREER Award (CCF-1053753). M.D.M.L. is supported by NSF fellowship GRFP DGE 0228243. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Data for HI2012, created by the Center for Cancer Systems Biology (CCSB) at the Dana-Farber Cancer Institute, are supported by the NHGRI of the US NIH, the Ellison Foundation and the Dana-Farber Cancer Institute Strategic Initiative.
Author information
Authors and Affiliations
Contributions
M.D.M.L., F.V., H.-T.W. and B.J.R. designed the HotNet2 algorithm. M.D.M.L., F.V., H.-T.W., J.R.D., J.V.E., J.L.T., Y.K. and B.J.R. performed pan-cancer network analysis, analyzed results and benchmarked algorithms. A.P., J.R.D., Y.C. and G.A.R. analyzed mutation clusters in genes. B.N., M.M. and L.D. provided MuSiC gene scores, assisted with figures and generated mutation validation data. M.S.L., G.G., A.G.-P., D.T. and N.L.-B. provided MutSigCV and Oncodrive gene scores. M.D.M.L., F.V., H.-T.W., J.R.D. and B.J.R. wrote the manuscript with input from all authors. B.J.R. conceived and supervised the project.
Corresponding author
Ethics declarations
Competing interests
A patent application related to this work has been filed with the US Patent and Trademark Office (USPTO).
Supplementary information
Supplementary Text and Figures
Supplementary Note and Supplementary Figures 1–30. (PDF 14283 kb)
Supplementary Tables 1–23 and 25–39
Supplementary Tables 1–23 and 25–39. (XLSX 219 kb)
Supplementary Table 24
Mutually exclusive and co-occurring test for pairwise genes within the pair of HotNet2 identified subnetworks across all pan-cancer samples. (XLSX 364 kb)
Rights and permissions
About this article
Cite this article
Leiserson, M., Vandin, F., Wu, HT. et al. Pan-cancer network analysis identifies combinations of rare somatic mutations across pathways and protein complexes. Nat Genet 47, 106–114 (2015). https://doi.org/10.1038/ng.3168
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3168
This article is cited by
-
Identification of VWA5A as a novel biomarker for inhibiting metastasis in breast cancer by machine-learning based protein prioritization
Scientific Reports (2024)
-
ICDM-GEHC: identifying cancer driver module based on graph embedding and hierarchical clustering
Complex & Intelligent Systems (2024)
-
Identifying cancer driver genes based on multi-view heterogeneous graph convolutional network and self-attention mechanism
BMC Bioinformatics (2023)
-
Identifying driver pathways based on a parameter-free model and a partheno-genetic algorithm
BMC Bioinformatics (2023)
-
Network embedding framework for driver gene discovery by combining functional and structural information
BMC Genomics (2023)