Abstract
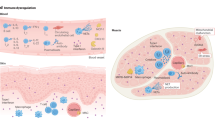
Autoimmune inflammatory myopathies, referred to as myositis, comprise a heterogeneous group of chronic inflammatory muscle diseases that present with various clinical phenotypes, histologic changes and autoantibodies, resulting in progressive inflammatory muscle damage and weakness. In up to 20% of myositis patients, particularly those with dermatomyositis, there is an association with cancer that is most frequently diagnosed within 1 year of presentation of myositis. Accumulating data show that autoantibodies in myositis target a specific group of intracellular molecules that are not muscle-specific in their expression. The striking association between autoantibodies recognizing ubiquitously expressed molecules and distinct clinical phenotypes suggests that the target tissues themselves might regulate and shape the phenotype-specific immune response in myositis. Studies indicate that changes in phenotype-specific autoantigens, such as altered structure, enhanced expression, and acquisition of adjuvant properties during various forms of cellular stress, apoptosis, and transformation, might be mechanistically important in this regard. This Review discusses these developments and highlights a central role of autoantigens themselves as a critical partner in driving autoimmune diseases, and the potential for their therapeutic manipulation. In addition, we will highlight insights that the cancer–autoimmunity interface in this group of diseases provides into the relationship between the anticancer immune response and autoimmune diseases.
Key Points
-
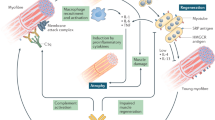
The specificity of the immune response in autoimmune inflammatory myopathies provides important insights into the disease mechanism, identifying the injured and healing muscle as an active participant in tissue damage through mutually amplifying interactions with the immune system
-
Damage-induced enhancement of autoantigen expression and adjuvant activity might contribute to an amplification loop in autoimmunity, in which tissue damage and healing provide fuel for further immune responses
-
Amplification cycles have an important role in self-sustaining phenotypes, and might be important targets of therapy
-
Relevant amplification loops in myositis include immune effector pathways generating muscle damage (e.g. the cytotoxic T lymphocyte granule pathway), antigen expression pathways, regeneration and differentiation pathways, and Toll-like receptor or interferon pathways
-
The association of malignancy with myositis and the shared antigen expression patterns in regenerating muscle and adenocarcinomas suggest that in some patients, myositis might represent a paraneoplastic syndrome reflecting an anticancer response that spreads to normal tissues
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dalakas MC (1991) Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med 325: 1487–1498
Love LA et al. (1991) A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 70: 360–374
Targoff IN (2000) Update on myositis-specific and myositis-associated autoantibodies. Curr Opin Rheumatol 12: 475–481
Rosen A and Casciola-Rosen L (1999) Autoantigens as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ 6: 6–12
Casciola-Rosen L et al. (2005) Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med 201: 591–601
Zhang Y et al. (1999) Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev 13: 1924–1935
Williams CJ et al. (2004) The chromatin remodeler Mi-2beta is required for CD4 expression and T cell development. Immunity 20: 719–733
Kashiwagi M et al. (2007) The chromatin remodeler Mi-2beta is required for establishment of the basal epidermis and normal differentiation of its progeny. Development 134: 1571–1582
Howard OM et al. (2002) Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J Exp Med 196: 781–791
Marshak-Rothstein A (2006) Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 6: 823–835
Christensen SR et al. (2006) Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25: 417–428
Lovgren T et al. (2004) Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 50: 1861–1872
Means TK et al. (2005) Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest 115: 407–417
Ronnblom L and Alm GV (2001) An etiopathogenic role for the type I IFN system in SLE. Trends Immunol 22: 427–431
Vollmer J et al. (2005) Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med 202: 1575–1585
Banchereau J and Pascual V (2006) Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25: 383–392
Lau CM et al. (2005) RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med 202: 1171–1177
Leadbetter EA et al. (2002) Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416: 603–607
Greenberg SA et al. (2005) Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol 57: 664–678
Bockenstedt LK et al. (1995) Self-peptides in the initiation of lupus autoimmunity. J Immunol 154: 3516–3524
Goebels N et al. (1996) Differential expression of perforin in muscle-infiltrating T cells in polymyositis and dermatomyositis. J Clin Invest 97: 2905–2910
Casciola-Rosen L et al. (1999) Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med 190: 815–826
Williams RC Jr (1959) Dermatomyositis and malignancy: a review of the literature. Ann Intern Med 50: 1174–1181
Barnes BE and Mawr B (1976) Dermatomyositis and malignancy. A review of the literature. Ann Intern Med 84: 68–76
Sigurgeirsson B et al. (1992) Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med 326: 363–367
Hill CL et al. (2001) Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 357: 96–100
Levine SM (2006) Cancer and myositis: new insights into an old association. Curr Opin Rheumatol 18: 620–624
Buchbinder R et al. (2001) Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med 134: 1087–1095
Amoura Z et al. (2005) Tumor antigen markers for the detection of solid cancers in inflammatory myopathies. Cancer Epidemiol Biomarkers Prev 14: 1279–1282
Nordlund JJ et al. (1983) Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol 9: 689–696
Darnell RB and Posner JB (2006) Paraneoplastic syndromes affecting the nervous system. Semin Oncol 33: 270–298
Gogas H et al. (2006) Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med 354: 709–718
Ge Q et al. (1995) Molecular analysis of a major antigenic region of the 240-kD protein of Mi-2 autoantigen. J Clin Invest 96: 1730–1737
Wade PA et al. (1999) Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nat Genet 23: 62–66
Hengstman GJ et al. (2006) Clinical characteristics of patients with myositis and autoantibodies to different fragments of the Mi-2 beta antigen. Ann Rheum Dis 65: 242–245
Miller T et al. (2002) Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry 73: 420–428
Utz PJ et al. (1998) The 72-kDa component of signal recognition particle is cleaved during apoptosis. J Biol Chem 273: 35362–35370
Oddis CV et al. (1992) Serum autoantibody to the nucleolar antigen PM/Scl. Clinical and immunogenetic associations. Arthritis Rheum 35: 1211–1217
Raijmakers R et al. (2003) The association of the human PM/Scl-75 autoantigen with the exosome is dependent on a newly identified N terminus. J Biol Chem 278: 30698–30704
Raijmakers R et al. (2004) PM/Scl-75 is the main autoantigen in patients with the polymyositis/scleroderma overlap syndrome. Arthritis Rheum 50: 565–569
Schilders G et al. (2007) Caspase-mediated cleavage of the exosome subunit PM/Scl-75 during apoptosis. Arthritis Res Ther 9: R12
Casciola-Rosen LA et al. (2001) The DNA mismatch repair enzyme PMS1 is a myositis-specific autoantigen. Arthritis Rheum 44: 389–396
Okada T et al. (2005) Immune responses to DNA mismatch repair enzymes hMSH2 and hPMS1 in patients with pancreatic cancer, dermatomyositis and polymyositis. Int J Cancer 116: 925–933
Rutjes SA et al. (1997) Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy. Clin Exp Immunol 109: 32–40
Espinosa A et al. (2006) The Sjogren's syndrome-associated autoantigen Ro52 is an E3 ligase that regulates proliferation and cell death. J Immunol 176: 6277–6285
Casciola-Rosen LA et al. (1995) DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J Exp Med 182: 1625–1634
Collis SJ et al. (2005) The life and death of DNA-PK. Oncogene 24: 949–961
Mimori T et al. (1981) Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis–scleroderma overlap. J Clin Invest 68: 611–620
Acknowledgements
This work was supported by NIH grants, by a Hugh and Renna Cosner scholarship in Translational Research, and by the Blum Kovler Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Suber, T., Casciola-Rosen, L. & Rosen, A. Mechanisms of Disease: autoantigens as clues to the pathogenesis of myositis. Nat Rev Rheumatol 4, 201–209 (2008). https://doi.org/10.1038/ncprheum0760
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0760
This article is cited by
-
Idiopathic Inflammatory Myopathies and Malignancy
Current Treatment Options in Rheumatology (2017)
-
Cell death, clearance and immunity in the skeletal muscle
Cell Death & Differentiation (2016)
-
Threonyl-tRNA synthetase overexpression correlates with angiogenic markers and progression of human ovarian cancer
BMC Cancer (2014)
-
Pathogenesis and prevention of rheumatic disease: focus on preclinical RA and SLE
Nature Reviews Rheumatology (2014)
-
Innate Immune-Response Mechanisms in Dermatomyositis: An Update on Pathogenesis, Diagnosis and Treatment
Drugs (2014)