Abstract
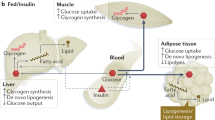
A wide range of genetically engineered murine models of type 2 diabetes have been created to try to understand the site of the primary defect in insulin action, and the relationship between insulin resistance and impaired β-cell function in diabetes. Genetic disruption of various aspects known to be important in diabetes has examined specific facets, including glucose sensing, transcription factors for the insulin gene, the insulin gene itself, insulin and insulin-like growth factor receptors, downstream signaling components and some mutations that increase insulin sensitivity. This article focuses on models that have given insight into insulin resistance and impaired insulin production, especially models that examine molecules involved in the signaling pathway downstream of insulin binding its receptor. These models recapitulate many features of human type 2 diabetes and, although they have emphasized the complexity of this disease, they offer numerous opportunities to characterize particular aspects and eventually fit them together to help delineate the human disease.
Key Points
-
The pathophysiology of type 2 diabetes mellitus includes β-cell dysfunction and insulin resistance
-
Mouse models that have specific gene-deletions are useful for studying disease processes such as diabetes
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Accili D (2004) Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic. Diabetes 53: 1633–1642
Permutt MA and Hattersley AT (2000) Searching for type 2 diabetes genes in the post-genome era. Trends Endocrinol Metab 11: 383–393
Nandi A et al. (2004) Mouse models of insulin resistance. Physiol Rev 84: 623–647
Accili D et al. (1996) Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet 12: 106–109
Michael MD et al. (2000) Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 6: 87–97
Bluher M et al. (2002) Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell 3: 25–38
Bluher M et al. (2003) Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299: 572–574
Guerra C et al. (2001) Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J Clin Invest 108: 1205–1213
Bruning JC et al. (1998) A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 2: 559–569
Bruning JC et al. (2000) Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125
Kulkarni RN et al. (1999) Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96: 329–339
Kulkarni RN et al. (2002) β-Cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter β-cell mass. Nat Genet 31: 111–115
Xuan S et al. (2002) Defective insulin secretion in pancreatic β cells lacking type 1 IGF receptor. J Clin Invest 110: 1011–1019
Okada T et al. (2007) Insulin receptors in β-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci U S A 104: 8977–8982
Laustsen PG et al. (2007) Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol Cell Biol 27: 1649–1664
Accili D (2001) A kinase in the life of the β cell. J Clin Invest 108: 1575–1576
Fernandez AM et al. (2001) Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev 15: 1926–1934
Zisman A et al. (2000) Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 6: 924–928
Tamemoto H et al. (1994) Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372: 182–186
Bruning JC et al. (1997) Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 88: 561–572
Kubota N et al. (2000) Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory β-cell hyperplasia. Diabetes 49: 1880–1889
Dong X et al. (2006) Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest 116: 101–114
Taguchi A et al. (2007) Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317: 369–372
Barbieri M et al. (2003) Glucose regulation and oxidative stress in healthy centenarians. Exp Gerontol 38: 137–143
Miller RA et al. (2002) Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell 1: 22–29
Bonkowski MS et al. (2006) Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A 103: 7901–7905
Liu SC et al. (1999) Insulin receptor substrate 3 is not essential for growth or glucose homeostasis. J Biol Chem 274: 18093–18099
Fantin VR et al. (2000) Mice lacking insulin receptor substrate 4 exhibit mild defects in growth, reproduction, and glucose homeostasis. Am J Physiol Endocrinol Metab 278: E127–E133
Cho H et al. (2001) Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB β). Science 292: 1728–1731
Garofalo RS et al. (2003) Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB β. J Clin Invest 112: 197–208
Katz EB et al. (1995) Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature 377: 151–155
Abel ED et al. (2001) Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409: 729–733
Crosson SM et al. (2003) PTG gene deletion causes impaired glycogen synthesis and developmental insulin resistance. J Clin Invest 111: 1423–1432
Poy MN et al. (2002) CEACAM1 regulates insulin clearance in liver. Nat Genet 30: 270–276
Moitra J et al. (1998) Life without white fat: a transgenic mouse. Genes Dev 12: 3168–3181
Shimomura I et al. (1998) Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev 12: 3182–3194
Duvillie B et al. (1997) Phenotypic alterations in insulin-deficient mutant mice. Proc Natl Acad Sci U S A 94: 5137–5140
Hong EG et al. (2007) Nonobese, insulin-deficient Ins2Akita mice develop type 2 diabetes phenotypes including insulin resistance and cardiac remodeling. Am J Physiol Endocrinol Metab 293: E1687–E1696
Stoy J et al. (2007) Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A 104: 15040–15044
Wellen KE and Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119
Harding HP and Ron D (2002) Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes 51 (Suppl 3): S455–S461
Zhang P et al. (2002) The PERK eukaryotic initiation factor 2 α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22: 3864–3874
Harding HP et al. (2001) Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell 7: 1153–1163
Froguel P and Velho G (1999) Molecular genetics of maturity-onset diabetes of the young. Trends Endocrinol Metab 10: 142–146
Grupe A et al. (1995) Transgenic knockouts reveal a critical requirement for pancreatic β cell glucokinase in maintaining glucose homeostasis. Cell 83: 69–78
Postic C et al. (1999) Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J Biol Chem 274: 305–315
Stoffers DA et al. (1997) Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet 15: 106–110
Stoffers DA et al. (1997) Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet 17: 138–139
Hani EH et al. (1999) Defective mutations in the insulin promoter factor-1 (IPF-1) gene in late-onset type 2 diabetes mellitus. J Clin Invest 104: R41–R48
Macfarlane WM et al. (1999) Missense mutations in the insulin promoter factor-1 gene predispose to type 2 diabetes. J Clin Invest 104: R33–R39
Ahlgren U et al. (1998) ß-Cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the β-cell phenotype and maturity onset diabetes. Genes Dev 12: 1763–1768
Johnson JD et al. (2003) Increased islet apoptosis in Pdx1+/− mice. J Clin Invest 111: 1147–1160
Butler AE et al. (2003) ß-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110
Naya FJ et al. (1997) Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev 11: 2323–2334
Yamagata K et al. (1996) Mutations in the hepatocyte nuclear factor-4α gene in maturity-onset diabetes of the young (MODY1). Nature 384: 458–460
Chen WS et al. (1994) Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev 8: 2466–2477
Li J et al. (2000) Mammalian hepatocyte differentiation requires the transcription factor HNF-4α. Genes Dev 14: 464–474
Gupta RK et al. (2005) The MODY1 gene HNF-4α regulates selected genes involved in insulin secretion. J Clin Invest 115: 1006–1015
Yamagata K et al. (1996) Mutations in the hepatocyte nuclear factor-1α gene in maturity-onset diabetes of the young (MODY3). Nature 384: 455–458
Pontoglio M et al. (1998) Defective insulin secretion in hepatocyte nuclear factor 1α-deficient mice. J Clin Invest 101: 2215–2222
Wang L et al. (2004) Selective deletion of the Hnf1β (MODY5) gene in β-cells leads to altered gene expression and defective insulin release. Endocrinology 145: 3941–3949
Nakae J et al. (2002) Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet 32: 245–253
Matsumoto M et al. (2007) Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor foxo1 in liver. Cell Metab 6: 208–216
Kitamura T et al. (2002) The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest 110: 1839–1847
Elchebly M et al. (1999) Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283: 1544–1548
Terauchi Y et al. (1999) Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 α subunit of phosphoinositide 3-kinase. Nat Genet 21: 230–235
Clement S et al. (2001) The lipid phosphatase SHIP2 controls insulin sensitivity. Nature 409: 92–97
Hirosumi J et al. (2002) A central role for JNK in obesity and insulin resistance. Nature 420: 333–336
Yuan M et al. (2001) Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293: 1673–1677
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
LeRoith, D., Accili, D. Mechanisms of Disease: using genetically altered mice to study concepts of type 2 diabetes. Nat Rev Endocrinol 4, 164–172 (2008). https://doi.org/10.1038/ncpendmet0729
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0729
This article is cited by
-
Circulating glucose levels inversely correlate with Drosophila larval feeding through insulin signaling and SLC5A11
Communications Biology (2018)
-
Chlorogenic acid/chromium supplement rescues diet-induced insulin resistance and obesity in mice
Nutrition & Metabolism (2015)
-
Drosophila glucome screening identifies Ck1alpha as a regulator of mammalian glucose metabolism
Nature Communications (2015)
-
A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity
Nature (2013)