Abstract
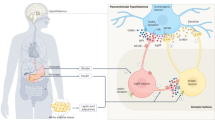
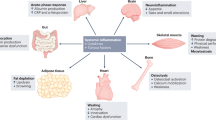
Cachexia is a process that accompanies many chronic diseases, and consists of a combination of wasting of lean body mass, increased energy expenditure, and a paradoxical loss of appetite. Cachexia both worsens quality of life and negatively affects treatment of the underlying disease. Conditions as diverse as cancer, renal failure, and heart failure show a remarkable similarity in their associated cachexia, exhibiting changes in metabolism and endocrinology, including marked increases in levels of cytokines that accompany these diseases. So far, it has been difficult to treat disease-associated cachexia successfully. One treatment that has shown promise in animal trials, however, involves antagonism of the central melanocortin system, an anorexigenic pathway in the hypothalamus and brainstem. Humans who have genetic mutations involving pro-opiomelanocortin or the melanocortin 4 receptor in this pathway exhibit increased appetite and increased lean body mass. Recent research has shown that in rodent models of cancer and renal failure, administration of melanocortin 4 receptor antagonists results in an attenuation of symptoms of cachexia, including maintenance of appetite, lean body mass, and basal energy expenditure. Although this research needs to be substantiated in humans, it provides a promising direction for treating the wasting that is associated with a variety of disease states.
Key Points
-
Cachexia is associated with chronic diseases and involves a paradoxical decrease in appetite, loss of lean body mass, and increase in total energy expenditure
-
Current therapies to treat cachexia have not shown significant effects in alleviating the associated loss of appetite and lean body mass
-
Many of the diseases associated with cachexia have a component of inflammation that seems to be involved in the development of these symptoms
-
Recent research has shown that the action of inflammatory cytokines on the melanocortin system might have a role in the anorexia and loss of lean body mass seen in cachexia
-
Use of small-molecule antagonists to components of the melanocortin system in animals has proven useful in alleviating the anorexia and loss of lean body mass associated with multiple models of chronic disease
-
Trials in humans with cachexia will be necessary to further delineate the usefulness of these molecules in alleviating these symptoms in the setting of chronic illness in humans
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Tisdale MJ (1997) Biology of cachexia. J Natl Cancer Inst 89: 1763–1773
Krude H et al. (1998) Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19: 155–157
Farooqi IS et al. (2003) Binge eating as a phenotype of melanocortin 4 receptor gene mutations. N Engl J Med 349: 606–609
Fearon KC (1992) The Sir David Cuthbertson Medal Lecture 1991. The mechanisms and treatment of weight loss in cancer. Proc Nutr Soc 51: 251–265
Qureshi AR et al. (1998) Factors predicting malnutrition in hemodialysis patients: a cross-sectional study. Kidney Int 53: 773–782
Vigano A et al. (2004) Quality of life and survival prediction in terminal cancer patients: a multicenter study. Cancer 101: 1090–1098
Anker SD et al. (1997) Wasting as independent risk factor for mortality in chronic heart failure. Lancet 349: 1050–1053
Wang AY et al. (2004) Resting energy expenditure and subsequent mortality risk in peritoneal dialysis patients. J Am Soc Nephrol 15: 3134–3143
Tisdale MJ (2002) Cachexia in cancer patients. Nat Rev Cancer 2: 862–871
Levine B et al. (1990) Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323: 236–241
Anker SD et al. (1997) Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation 96: 526–534
Torre-Amione G et al. (1996) Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 27: 1201–1206
Wigmore SJ et al. (1994) IL-8 and IL-6 are produced constitutively by human pancreatic cancer cell lines [abstract]. Gut 35 (Suppl 5): 539
Deans C and Wigmore SJ (2005) Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care 8: 265–269
Kaysen GA et al. (1995) Mechanisms of hypoalbuminemia in hemodialysis patients. Kidney Int 48: 510–516
Cone R (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578
Lubrano-Berthelier C et al. (2004) A homozygous null mutation delineates the role of the melanocortin-4 receptor in humans. J Clin Endocrinol Metab 89: 2028–2032
Popovic V and Duntas LH (2005) Brain somatic cross-talk: ghrelin, leptin and ultimate challengers of obesity. Nutr Neurosci 8: 1–5
Batterham RL et al. (2002) Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654
Hillhouse EW and Mosley K (1993) Peripheral endotoxin induces hypothalamic immunoreactive interleukin-1β in the rat. Br J Pharmacol 109: 289–290
Sergeyev V et al. (2001) Effect of LPS administration on the expression of POMC, NPY, galanin, CART and MCH mRNAs in the rat hypothalamus. Brain Res Mol Brain Res 90: 93–100
Huang et al. (1999) Role of central melanocortins in endotoxin-induced anorexia. Am J Physiology 276: R864–R871
Marks DL et al. (2001) Role of the central melanocortin system in cachexia. Cancer Res 61: 1432–1438
Cheung W et al. (2005) Role of leptin and melanocortin signaling in uremia-associated cachexia. J Clin Invest 115: 1659–1665
Markison S et al. (2005) The regulation of feeding and metabolic rate and the prevention of murine cancer cachexia with a small-molecule melanocortin-4 receptor antagonist. Endocrinology 146: 2766–2773
Vos T et al. (2004) Identification of 2-{2-[2-(5-bromo-2-methoxyphenyl)-ethyl]-3-fluorophenyl}-4,5-dihydro-1H-imidazole (ML00253764), a small molecule melanocortin 4 receptor antagonist that effectively reduces tumor-induced weight loss in a mouse model. J Med Chem 47: 1602–1604
Nicholson JR et al. (2006) Peripheral administration of a melanocortin 4-receptor inverse agonist prevents loss of lean body mass in tumor-bearing mice. J Pharmacol Exp Ther 317: 771–777
Pierroz D et al. (2002) Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes 51: 1337–1345
Bluher S et al. (2004) Responsiveness to peripherally administered melanocortins in lean and obese mice. Diabetes 53: 82–90
Lundholm K et al. (1994) Anti-inflammatory treatment may prolong survival in undernourished patients with metastatic solid tumors. Cancer Res 54: 5602–5606
Loprinzi CL et al. (1999) Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol 17: 3299–3306
Wigmore SJ et al. (1997) Down-regulation of the acute-phase response in patients with pancreatic cancer cachexia receiving oral eicosapentaenoic acid is mediated via suppression of interleukin-6. Clin Sci (Lond) 92: 215–221
Smith HJ et al. (1999) Effect of a cancer cachectic factor on protein synthesis/degradation in murine C2C12 myoblasts: modulation by eicosapentaenoic acid. Cancer Res 59: 5507–5513
Fearon KC et al. (2003) Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut 52: 1479–1486
Jatoi A et al. (2004) An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol 22: 2469–2476
Simons JP et al. (1998) Effects of medroxyprogesterone acetate on food intake, body composition, and resting energy expenditure in patients with advanced, nonhormone-sensitive cancer: a randomized, placebo-controlled trial. Cancer 82: 553–560
Strang P (1997) The effect of megestrol acetate on anorexia, weight loss and cachexia in cancer and AIDS patients (review). Anticancer Res 17: 657–662
Johannsson G et al. (1999) Double-blind, placebo-controlled study of growth hormone treatment in elderly patients undergoing chronic hemodialysis: anabolic effect and functional improvement. Am J Kidney Dis 33: 709–717
Hansen TB et al. (2000) Influence of growth hormone on whole body and regional soft tissue composition in adult patients on hemodialysis. A double-blind, randomized, placebo-controlled study. Clin Nephrol 53: 99–107
Johansen KL et al. (1999) Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. JAMA 281: 1275–1281
Anker SD et al. (2003) Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet 361: 1077–1083
Parissis JT et al. (2005) Effects of levosimendan on markers of left ventricular diastolic function and neurohormonal activation in patients with advanced heart failure. Am J Cardiol 96: 423–426
Anker SD and Sharma R (2002) The syndrome of cardiac cachexia. Int J Cardiol 85: 51–66
Wisse BE et al. (2001) Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology 142: 3292–3301
Marks DL et al. (2003) Differential role of melanocortin receptor subtypes in cachexia. Endocrinology 144: 1513–1523
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Daniel L Marks serves as a scientific consultant for Neurocrine Biosciences, Inc. and Ipsen, Inc. MD DeBoer declared he has no competing interests.
Rights and permissions
About this article
Cite this article
DeBoer, M., Marks, D. Therapy insight: use of melanocortin antagonists in the treatment of cachexia in chronic disease. Nat Rev Endocrinol 2, 459–466 (2006). https://doi.org/10.1038/ncpendmet0221
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0221
This article is cited by
-
Ghrelin and cachexia in chronic kidney disease
Pediatric Nephrology (2013)
-
Partial normalization of pubertal timing in female mice with DSS colitis treated with anti-TNF-α antibody
Journal of Gastroenterology (2012)
-
The orally active melanocortin-4 receptor antagonist BL-6020/979: a promising candidate for the treatment of cancer cachexia
Journal of Cachexia, Sarcopenia and Muscle (2011)
-
Hormonal Regulators of Appetite
International Journal of Pediatric Endocrinology (2009)
-
The need for a standardized definition for cachexia in chronic illness
Nature Clinical Practice Endocrinology & Metabolism (2006)