Abstract
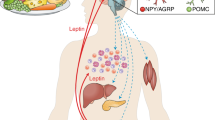
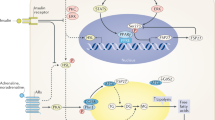
Since adipocytes express specific receptors for pituitary hormones and hypothalamic releasing factors, adipose tissue has to be regarded as a fast-acting endocrine gland under the control of the brain. Expanding on this suggestion, the existence and clinical impact of a hypothalamic–pituitary–adipose axis is reviewed. The term 'adipotropins' is introduced in order to describe pituitary and hypothalamic hormones or releasing factors that directly target adipocytes by their specific receptors.
Key Points
-
Adipocytes express the specific receptors for nearly all known pituitary hormones and hypothalamic releasing factors
-
On this basis, the existence of a hypothalamic–pituitary–adipose axis has to be considered
-
Pituitary hormones and hypothalamic releasing factors can directly influence adipokine secretion and a wide variety of adipocyte functions
-
The name 'adipotropins' is suggested to characterize pituitary hormones and hypothalamic releasing factors that act on adipocytes via their specific receptors
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wang Q et al. (1997) Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis in the rat. Diabetes 46: 335–341
Qi Y et al. (2004) Adiponectin acts in the brain to decrease body weight. Nat Med 10: 524–529
Schäffler A et al. (2005) Hypothesis paper: brain talks with fat—evidence for a hypothalamic–pituitary–adipose axis? Neuropeptides 39: 363–367
Flint DJ et al. (2003) Effects of growth hormone and prolactin on adipose tissue development and function. Pituitary 6: 97–102
Ling C et al. (2003) Identification of functional prolactin (PRL) receptor gene expression: PRL inhibits lipoprotein lipase activity in human white adipose tissue. J Clin Endocrinol Metab 88: 1804–1808
Viengchareun S et al. (2004) Prolactin potentiates insulin-stimulated leptin expression and release from differentiated brown adipocytes. J Mol Endocrinol 33: 679–691
Ling C and Billig H (2001) PRL receptor-mediated effects in female mouse adipocytes: PRL induces suppressors of cytokine signaling expression and suppresses insulin-induced leptin production in adipocytes in vitro. Endocrinology 142: 4880–4890
Freemark M et al. (2001) Body weight and fat deposition in prolactin receptor-deficient mice. Endocrinology 142: 532–537
Nilsson L et al. (2005) Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem Biophys Res Commun 331: 1120–1126
Hogan JC and Stephens JM (2005) The regulation of fatty acid synthase by STAT5A. Diabetes 54: 1968–1975
Gualillo O et al. (1999) Prolactin stimulates leptin secretion by rat white adipose tissue. Endocrinology 140: 5149–5153
Ling C et al. (2001) Increased resistin expression in the adipose tissue of male prolactin transgenic mice and in male mice with elevated androgen levels. FEBS Lett 507: 147–150
Wu SL et al. (1999) Demonstration of thyrotropin receptor mRNA in orbital fat and eye muscle tissues from patients with Graves' ophthalmopathy by in situ hybridization. J Endocrinol Invest 22: 289–295
Ludgate M et al. (1998) The thyrotropin receptor in thyroid eye disease. Thyroid 8: 411–413
Valyasevi RW et al. (2002) Stimulation of adipogenesis, peroxisome proliferator-activated receptor-gamma (PPARγ), and thyrotropin receptor by PPARγ agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab 87: 2352–2358
Valyasevi RW et al. (1999) Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab 84: 2557–2562
Sorisky A et al. (2000) TSH receptor in adipose cells. Horm Metab Res 32: 468–474
Shimura H et al. (1998) Analysis of differentiation-induced expression mechanisms of thyrotropin receptor gene in adipocytes. Mol Endocrinol 12: 1473–1486
Bell A et al. (2002) TSH signaling and cell survival in 3T3-L1 preadipocytes. Am J Physiol Cell Physiol 283: C1056–C1064
Menendez C et al. (2003) TSH stimulates leptin secretion by a direct effect on adipocytes. J Endocrinol 176: 7–12
Shintani M et al. (1999) Thyrotropin decreases leptin production in rat adipocytes. Metabolism 48: 1570–1574
Yaturu S et al. (2004) Changes in adipocyte hormones leptin, resistin, and adiponectin in thyroid dysfunction. J Cell Biochem 93: 491–496
Santini F et al. (2004) Serum concentrations of adiponectin and leptin in patients with thyroid dysfunctions. J Endocrinol Invest 27: RC5–RC7
Janson A et al. (1998) Presence of thyrotropin receptor in infant adipocytes. Pediatr Res 43: 555–558
Janson A et al. (1995) Effects of stimulatory and inhibitory thyrotropin receptor antibodies on lipolysis in infant adipocytes. J Clin Endocrinol Metab 80: 1712–1716
Heufelder AE (2000) Pathogenesis of ophthalmopathy in autoimmune thyroid disease. Rev Endocr Metab Disord 1: 87–95
Jyonouchi SC et al. (2001) Interleukin-6 stimulates thyrotropin receptor expression in human orbital preadipocyte fibroblasts from patients with Graves' ophthalmopathy. Thyroid 11: 929–934
Valyasevi RW et al. (2001) Effect of tumor necrosis factor-α, interferon-γ, and transforming growth factor-β on adipogenesis and expression of thyrotropin receptor in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab 86: 903–908
Bell A et al. (2003) TSH stimulates IL-6 secretion from adipocytes in culture. Arterioscler Thromb Vasc Biol 23: 65–66
Bahn RS et al. (1998) Thyrotropin receptor expression in cultured Graves' orbital preadipocyte fibroblasts is stimulated by thyrotropin. Thyroid 8: 193–196
Starkey K et al. (2003) Peroxisome proliferator-activated receptor-γ in thyroid eye disease: contraindication for thiazolidinedione use? J Clin Endocrinol Metab 88: 55–59
Vikman K et al. (1991) Expression and regulation of growth hormone (GH) receptor messenger ribonucleic acid (mRNA) in rat adipose tissue, adipocytes, and adipocyte precursor cells: GH regulation of GH receptor mRNA. Endocrinology 129: 1155–1161
Hellgren G et al. (2001) Growth hormone receptor interaction with JAK proteins differs between tissues. J Interferon Cytokine Res 21: 75–83
Barnard R et al. (1990) Soluble forms of the rabbit adipose tissue and liver growth hormone receptors are antigenically identical, but the integral membrane forms differ. Biochem J 267: 471–477
Nam SY and Lobie PE (2000) The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes Rev 1: 73–86
Nam SY and Marcus C (2000) Growth hormone and adipocyte function in obesity. Horm Res 53 (Suppl 1): 87–97
Wabitsch M et al. (1996) Mitogenic and antiadipogenic properties of human growth hormone in differentiating human adipocyte precursor cells in primary culture. Pediatr Res 40: 450–456
Kralisch S et al. (2005) Hormonal regulation of the novel adipocytokine visfatin in 3T3-L1 adipocytes. J Endocrinol 185: R1–R8
Delhanty PJ et al. (2002) Growth hormone rapidly induces resistin gene expression in white adipose tissue of spontaneous dwarf (SDR) rats. Endocrinology 143: 2445–2448
Cone RD et al. (1996) The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res 51: 287–317
Boston BA and Cone RD (1996) Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line. Endocrinology 137: 2043–2050
Norman D et al. (2003) ACTH and α-MSH inhibit leptin expression and secretion in 3T3-L1 adipocytes: model for a central-peripheral melanocortin-leptin pathway. Mol Cell Endocrinol 200: 99–109
Cho KJ et al. (2005) Signaling pathways implicated in α-melanocyte stimulating hormone-induced lipolysis in 3T3-L1 adipocytes. J Cell Biochem 96: 869–878
Morimoto C et al. (1998) Antilipolytic actions of insulin on basal and hormone-induced lipolysis in rat adipocytes. J Lipid Res 39: 957–962
Izawa T et al. (1994) Increase in cytosolic free Ca2 in corticotropin-stimulated white adipocytes. Am J Physiol 266 (3 Pt 1): E418–E426
Xue B et al. (1998) The agouti gene product inhibits lipolysis in human adipocytes via a Ca2-dependent mechanism. FASEB J 12: 1391–1396
Shirakura S et al. (1990) Activation of glucose transport by activatory receptor agonists of adenlyate cyclase in rat adipocytes. Comp Biochem Physiol A 97: 81–86
Marette A et al. (1990) Mechanism of norepinephrin stimulation of glucose transport in isolated rat brown adipocytes. Int J Obes 14: 857–867
Kuroda M et al. (1987) Regulation of insulin-stimulated glucose transport in the isolated rat adipocyte. cAMP-independent effects of lipolytic and antilipolytic agents. J Biol Chem 262: 245–253
Friedberg M et al. (2003) Modulation of 11 β-hydroxysteroid dehydrogenase type 1 in mature human subcutaneous adipocytes by hypothalamic messengers. J Clin Endocrinol Metab 88: 385–393
Bradley RL et al. (2005) Neuropeptides, including neuropeptide Y and melanocortins, mediate lipolysis in murine adipocytes. Obes Res 13: 653–661
Boston BA (1999) The role of melanocortins in adipocyte function. Ann NY Acad Sci 885: 75–84
Hoggard N et al. (2004) Regulation of adipose tissue leptin secretion by α-melanocyte-stimulating hormone and agouti-related protein: further evidence of an interaction between leptin and the melanocortin signalling system. J Mol Endocrinol 32: 145–153
Boland D and Goren HJ (1987) Binding and structural properties of oxytocin receptors in isolated rat epididymal adipocytes. Regul Pept 18: 7–18
Gimpl G and Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81: 629–683
Egan JJ et al. (1990) Insulin, oxytocin, and vasopressin stimulate protein kinase C activity in adipocyte plasma membranes. Proc Natl Acad Sci USA 87: 1052–1056
Krieger-Brauer HI and Kather H (1995) The stimulus-sensitive H2O2-generating system present in human fat-cell plasma membranes is multireceptor-linked and under antagonistic control by hormones and cytokines. Biochem J 307: 543–548
Axelrod L et al. (1986) Prostacyclin production by isolated rat adipocytes: evidence for cyclic adenosine 3',5'-monophosphate-dependent and independent mechanisms and for a selective effect of insulin. Endocrinology 119: 2233–2239
Cheng K and Larner J (1985) Unidirectional actions of insulin and Ca2-dependent hormones on adipocyte pyruvate dehydrogenase. J Biol Chem 260: 5279–5285
Blackmore PF and Augert G (1989) Effect of hormones on cytosolic free calcium in adipocytes. Cell Calcium 10: 561–567
Hernandez A and Obregon MJ (1995) Presence of growth factors-induced type III iodothyronine 5-deiodinase in cultured rat brown adipocytes. Endocrinology 136: 4543–4550
Seres J et al. (2004) Corticotropin-releasing hormone system in human adipose tissue. J Clin Endocrinol Metab 89: 965–970
Hochberg Z et al. (2004) Hypothalamic regulation of adiposity: the role of 11β-hydroxysteroid dehydrogenase type 1. Horm Metab Res 36: 365–369
Murdoch WJ (1995) Immunolocalization of a gonadotropin-releasing hormone receptor site in murine endometrium that mediates apoptosis. Cell Tissue Res 282: 527–529
Dodt C et al. (2003) Sympathetic control of white adipose tissue in lean and obese humans. Acta Physiol Scand 177: 351–357
Turtzo LC et al. (2002) Completing the loop: neuron-adipocyte interactions and the control of energy homeostasis. Horm Metab Res 34: 607–615
Grunfeld C et al. (1985) Characterization of adrenocorticotropin receptors that appear when 3T3-L1 cells differentiate into adipocytes. Endocrinology 116: 113–117
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Schäffler, A., Schölmerich, J. & Buechler, C. The role of 'adipotropins' and the clinical importance of a potential hypothalamic–pituitary–adipose axis. Nat Rev Endocrinol 2, 374–383 (2006). https://doi.org/10.1038/ncpendmet0197
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0197
This article is cited by
-
Regulation of NR4A by nutritional status, gender, postnatal development and hormonal deficiency
Scientific Reports (2014)
-
The melanocortin 1 receptor (MC1R) inhibits the inflammatory response in Raw 264.7 cells and atopic dermatitis (AD) mouse model
Molecular Biology Reports (2013)