Abstract
The crystalline stereocomplexed polycarbonates can be prepared by mixing enantiopure polymers with opposite configuration, which derived from the asymmetric copolymerization with CO2 using enantiopure catalyst or/and chiral epoxides. Herein, we develop a powerful strategy for producing crystalline intramolecular stereocomplexed polycarbonates from racemic catalysts, which possess similar thermal stability and crystalline behaviour in comparison with the stereocomplexes by mixing opposite enantiopure polymers. Living polymer chains shuttle between catalyst molecules with different configurations to produce diastereomeric active species which is suggested to be responsible for the formation of isotactic multiblock polycarbonates in racemic bimetallic cobalt catalyst-mediated stereoselective copolymerization of CO2 and meso-epoxides. Solid-state NMR spectroscopy study suggests that the interaction in the carbonyl and methine regions is responsible for the strong crystallization capacity and compact package structure in the crystalline polycarbonates.
Similar content being viewed by others
Introduction
The alternating copolymerization of carbon dioxide (CO2) with epoxides to provide degradable polycarbonates is widely regarded as a promising green process worthy of intense scrutiny since it utilizes CO2 as C1 feedstock, an abundant and renewable carbon resource1,2,3,4,5,6. In the past decade, numerous homegeneous and hetereogeneous catalyst systems were developed for this transformation for achieving enhanced activity and high molecular weight7,8,9,10,11,12,13,14,15,16,17,18,19,20,21. Unfortunately, most of the previously reported CO2-based polycarbonates are amorphous, with a low glass transition temperature (Tg) <50 °C, significantly confining their applications, especially as structural materials.
It is generally known that the physical properties of a polymer are determined not only by the monomer structure, its molecular weight and polydispersity, but also by the relative stereochemistry (the spatial arrangement of atoms or groups in a polymeric unit) of adjacent locations in the polymeric chains. A representative example is the widely studied polypropylene. The isotactic polypropylene is a typical semicrystalline material, possessing a melting temperature (Tm) of 130−175 °C, dependent on the isotacticity, while the amorphous polypropylene is a viscous polymer at ambient temperature with a Tg of ∼0 °C (ref. 22). For CO2-based polycarbonates, in comparison with their amorphous structure, the crystalline forms should show improved thermal and mechanical properties, due to the high stereoregularity. In the recent contributions, some crystalline polycarbonates were prepared by stereospecific copolymerization of CO2 and epoxides using enantiopure metal-complex catalysts23,24,25. Notably, isotactic polycarbonates from meso-epoxides showed high levels of crystallinity, possessing Tms of 179−273 °C, dependent on the structure of the epoxides26,27,28,29,30. Interestingly, the cocrystallinzation of amorphous isotactic polycarbonates having opposite configurations and identical structures was observed to form crystalline stereocomplexes31,32, which show enhanced thermal stability and new crystalline behaviour, significantly distinct from the component enantiomers. These discoveries open up a new way to prepare various semicrystalline materials having a wide variety of physical properties. Nevertheless, these crystalline materials all originate from chiral isotactic polycarbonates prepared by the enantiopure metal-complex-mediated CO2/epoxides copolymerization. As a consequence, these processes are far away from practical applications, due to the high cost of chiral catalysts. Therefore, the exploration of the synthesis of crystalline CO2-based polycarbonates from racemic catalyst and rac- or meso-epoxides is highly desirable.
Herein, we report an approach for the synthesis of intramolecular stereocomplexed polycarbonates by stereoselective copolymerization CO2 with meso-epoxides using racemic dinuclear Co(III) complex as catalyst (Fig. 1), which possess similar thermal stability and crystalline behaviour in comparison with the stereocomplexes by mixing opposite enantiopure polymers.
Results
Synthesis of crystalline polycarbonates from 3,5-dioxaepoxide
For the alternating copolymerization of CO2 with meso-epoxides mediated by racemic isotactic catalyst systems, three possible microstructures might be observed in the resultant copolymers, dependent on the copolymerization chain growth rate (Rg) and polymer chain-transfer rate (Rt) (Fig. 2). Only when Rg is significantly higher than Rt, the racemic isotactic catalyst-mediated copolymerization reaction provides isotactic polycarbonates or isotactic multiblock polymers. The racemic–(SalBinap)–AlOiPr complex has been demonstrated to be very effective for the ring-opening polymerization of racemic lactide, affording the crystalline, stereoblock polymers33. Coates and co-workers presented the first report for synthesizing a broad range of highly isotactic polyethers via the enantioselective polymerization of racemic epoxides using racemic catalyst34,35,36. In this system, (S)-binaphthol linked dinuclear cobalt complex predominantly catalysed the ring-opening polymerization of (S)-epoxides to afford (S)-polyethers, while the polymerization of (R)-epoxides only concerned (R)-binaphthol linked catalyst to provide (R)-polyethers. The resultant mixture of (S)- and (R)-polyethers are highly isotactic, and most of them display high Tm values. Nevertheless, no cocrystallization occurs in the resultant mixture, in comparison with (S)- or (R)-polyethers.
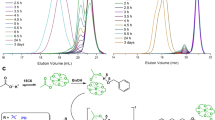
Initially, racemic dinuclear Co(III) catalyst 1 was first applied to the alternating copolymerization of CO2 with 4,4-dimethyl-3,5,8-trioxabicyclo[5.1.0]octane (CXO), a meso-epoxide with high reactivity. Previous study demonstrated that isotactic polycarbonates from CXO/CO2 enantioselective copolymerization (PCXC) exhibited a melting temperature of 242 °C (ref. 28). Notably, when (R)-PCXC and (S)-PCXC are mixed in equivalent amounts, cocrystallization occurs, affording a stereocomplex with a new crystalline behaviour, significantly different from that of the sole configuration PCXC32. As a consequence, it was expected to form crystalline stereocomplex from racemic dinuclear Co(III) catalyst 1-mediated CXO/CO2 copolymerization, if (R,R,R,R)-1 in the racemic catalyst system predominantly produces enantiopure (R)-PCXC, and (S,S,S,S)-1 mainly affords enantiopure (S)-PCXC. We delightedly found that the CO2/CXO copolymerization using racemic-1 in conjunction with PPN–DNP (PPN, bis(triphenylphosphine)iminium; DNP, 2,4-dinitrophenoxide) at 25 °C and 1.5 MPa CO2 pressure yielded highly crystalline polymer with a turnover frequency of 199 h−1 (Table 1, entry 1). On the basis of fast-scan chip-calorimeter measurement (Flash DSC), high-melting endothermic peak was found at 340 °C, which is significantly different from the sole configuration (R)- or (S)-PCXC (Fig. 3, top, plots A and C). Also, the wide-angle X-ray diffraction study confirms its semicrystalline structure. Several diffraction peaks appearing at 2θ equal to 6.4°, 13.3°, 16.0°, 17.9°, 20.5° and 23.0° (d=13.80, 6.65, 5.53, 4.95, 4.33 and 3.86, respectively) are consistent with the stereocomplexed PCXC by mixing enantiopure isotactic (R)- and (S)-PCXC in equivalent amount, but significantly distinct from that of the individual enantiomers (Fig. 3, bottom). Moreover, 13C NMR study demonstrates that no obvious difference was observed in the peaks corresponding to carbonyl and methine region between PCXCs prepared by mixing opposite enantiomers and by using rac-1, but significantly different from the atactic analogue (Fig. 4).
(A) (R)- or (S)-PCXC with 99% ee; (B) stereocomplexed PCXC prepared by mixing (R)- and (S)-polymres with 1:1 mass ratio; (C) PCXC prepared from rac-1/PPN–DNP catalysed CXO/CO2 copolymerization (Table 1, entry 1). The samples were crystallized isothermally at 180 °C for 2 h and samples of B and C in DSC thermograms was determined by FSC.
(a) Atactic PCXC; (b) enantiopure isotactic (S)-PCXC; (c) stereocomplexed PCXC prepared by mixing (R)- and (S)-polymers in 1:1 mass ratio; (d) PCXC prepared from rac-1/PPN–DNP mediated CXO/CO2 copolymerization (Table 1, entry 1).
Previously, we have demonstrated that isotactic (R)-PCXC or (S)-PCXC were easily dissolved in various organic solvent, such as dimethylsulphoxide and tetrahydrofuran (THF), while stereocomplexed PCXC prepared from mixing equivalent (R)- and (S)-PCXC had no solubility in these solvents. It was found that the copolymer formed from the racemic catalyst system also had no solubility in both dimethylsulphoxide and THF, suggesting the formation of the stereocomplex. Furthermore, methanol was added as a chain-transfer reagent to the copolymerization mediated by rac-1/PPN–DNP catalyst system at the identical reaction conditions. We discovered that the resultant polymers were also crystallizable, although the melting temperature was decreased to a certain extent (Table 1, entries 1–4). For example, a Tm of 221 °C was found in the copolymer produced from rac-1/PPN–DNP catalyst system in the presence of 100 equivalents of MeOH, which is 119 °C lower than the PCXC resulted from the same catalyst in the absence of MeOH (Supplementary Fig. 1). The addition of methanol also resulted in the significant decrease in the copolymerization rate. In addition, a decrease in Mn is very obvious, and thereby causing their dissolvable in THF and dimethylsulphoxide. 13C NMR analysis show that the peaks corresponding to carbonyl and methine region were found to be splitted when the reaction was carried out in the presence of methanol (Supplementary Fig. 2), suggesting a decrease in stereoregularity.
It is worth noting that the intensities of various diffraction peaks in the copolymer sample from racemic catalyst are obviously lower than the stereocomplexed PCXC obtained from the 1:1 mixture of the opposite enantiomers (Fig. 3, bottom, plots B and C). In addition, the melting endothermic peak is also slightly lower than that of the stereocomplexed PCXC with a Tm of 347 °C (Fig. 3, top, plots B and C). These results suggest that the crystallinity of the resultant PCXC from racemic-1 catalyst system is significantly lower than that of the stereocomplexed PCXC consisted of the mixed opposite enantiomers. We tentatively assume that the copolymer from racemic-1 catalyst system is an isotactic multiblock polymer. The reduced isotacticity originates from the stereoerrors in the copolymer caused by the polymer growth-chain transfer between (R,R,R,R)-1 and (S,S,S,S)-1 during the copolymerization. As a result, the intramolecular cocrystallization of the isotactic multiblock-PCXC from racemic-1 catalyst system predominantly contributes the formation of intramolecular stereocomplexed polycarbonates.
Solid state NMR spectroscopy is a powerful tool for studying the polymer segment, structure and dynamics. Macromolecular motions covering a wide range of time scales have long been considered to affect the physical and mechanical properties37,38,39,40. Usually, polymers in amorphous state show high-amplitude motions, especially for the temperature above the Tgs. As previously mentioned, there are huge differences in physical properties for the amorphous, enantiopure and stereocomplexed-PCXCs in solid state, such as solubility, melting and crystalline behaviour. In the present study, solid state NMR spectroscopy was also employed for studying the difference in microstructure of various PCXCs (Fig. 5). Spin-lattice relaxation time (T1) was measured for each carbon atom in four representative PCXC samples under the cross-polarisation condition by application of the saturation recovery-based sequence (Table 2). We discovered that the T1 value of carbonyl region for the enantiopure PCXC was longer than the amorphous state. Especially, the T1 value of carbonyl region for the stereocomplexed PCXC is up to 223 s, which is 175 s longer than the amorphous state and 131 s longer than enantiopure-PCXCs, in accordance with its much stronger crystallization capacity and compact package structure. Notably, it was demonstrated that PCXC resulted from racemic-1 (multiblock-PCXC) possessed a similar structure with the stereocomplexed PCXC, because its T1 value in carbonyl region also reached to 156 s. There is an interesting information for methine region, which was found to be splitted to three peaks for multiblock-PCXCs, and two peaks for stereocomplexed-PCXCs. The T1 values are 165 and 150 s for stereocomplexed-PCXCs, in agreement with 110 and 116 s for multiblock-PCXCs. However, no split was observed in the methine region for amorphous or enantiopure-PCXCs, in which T1 values are 38 and 36 s, respectively. Interestingly, T1 value for the middle peak in the methine region for multiblock-PCXCs is 50 s, corresponding to the methine region for amorphous or enantiopure-PCXCs. We tentatively ascribe it to the minor glassy state in the crystalline domains, which originated from the stereoerrors in the copolymer caused by the polymer growth-chain transfer between (R,R,R,R)-1 and (S,S,S,S)-1 during the copolymerization. Nevertheless, T1 values of quaternary and primary carbon for amorphous PCXCs were very similar to those measured for stereocomplexed- and multiblock-PCXCs, indicating that they are more inclined to motion and the energy can be released more easily.
Mechanistic study for racemic-1 mediated copolymerization
Indeed, 13C NMR spectra of carbonyl and methine regions in Fig. 4 did not give the accurate isotacticity of the multiblock-PCXCs originated from the racemic catalyst system. In order to confirm the formation of the isotactic multiblock structure, cyclohexene oxide (CHO) was chosen as a model monomer of meso-epoxides for testing the stereoregularity of its CO2 copolymer produced by racemic Co(III) complexes, since the microstructure of poly(cyclohexene carbonate) (PCHC) was well-characterized41,42,43. Indeed, CHO also has relatively high reactivity in copolymerizing with CO2 catalysed by both mono- and di-nuclear Co(III) complexes in the presence of a nucleophilic cocatalyst. In previous study, we have demonstrated that for the mono-nuclear Co(III) complex-mediated CO2/epoxide copolymerization, the dissociation of the propagating carboxylate from the metal centre is a much faster process than propagation, and the free propagating carboxylate can also act as a nucleophile for attack at a cobalt-coordinated epoxide, so the binary catalyst system of racemic mono-nuclear Co(III)–Salen complex and PPN–DNP for CHO/CO2 copolymerization provided atactic PCHC18. On the contrary, the racemic dinuclear Co(III) complex 1 gave isotactic-enriched PCHC, based on the 13C NMR analysis. However, the isotacticity is obviously lower than that obtained from enantiopure dinuclear Co(III) complex. This means the occurrence of the copolymer-chain transfer between two kinds of catalyst molecules with different configurations.
Previously, various stereoregular PCHCs tetrad and triad sequences have been assigned in the 13C NMR spectrum42,43. In keeping with the previously established conventions in this field, it is important to note that [m] and [r] assignments used herein represent the relative stereochemistry of the carbons of the cyclohexene carbonate units (Supplementary Fig. 3). By synthesizing model poly(cyclohexene carbonate) oligomers or using Bernoullian statistical methods, all [mmm] and [mmr] tetrads were correlated to one central resonance at 153.7 p.p.m. and the remaining r-centred tetrads resided in the 153.3–153.0 p.p.m. range. The carbonyl region of the 13C NMR spectra of various PCHCs resulted from different catalysts or conditions is shown in Fig. 6 (The relationship between the tetrad sequences and the polymer microstructures was described in Supplementary Fig. 3). On the basis of the peaks assigned to the appropriate tetrads in accordance with the literature, the PCHC with a Pm of 0.84 obtained from (S,S,S,S)-1/PPN–DNP catalyst system revealed a 13C NMR spectrum with two distinct resonances at 153.70 and 153.04 p.p.m. assigning to [mmm+mmr] and [mrr] tetrads, respectively (Fig. 6, plot B). Especially, the peak at 153.04 p.p.m. for PCHC with a Pm of 0.96 decreased significantly (Fig. 6, plot A). The r-centred [mrr] tetrad was produced by the errors in the chain growth (mismatched monomer was incorporated) and then corrected by the chiral environment that is constructed by the ligand around the metal centre through an enantiomorphic site control. However, for polycarbonates resulted from racemic-1 (Fig. 6, plot C), a peak at 153.22 p.p.m. was discovered, corresponding to [mrm] tetrad, significantly distinct from PCHC resulted from (S,S,S,S)-1 with the same Pms (Fig. 6, plot B). Moreover, because of the formation of [mmr] tetrads, the peak corresponding to m-centred tetrads of polycarbonates resulted from racemic-1 become broaden in comparison with the PCHC with the identical stereoregularity. The 13C NMR spectra of methylene region also confirmed the results (Supplementary Fig. 4). The presence of a small [mrr] peak also suggests that a minimal amount of the unpreferred enantiomer is incorporated into the chain at a level, consistent with the PCHCs described in plots A and B.
On the basis of [mrm] tetrad in 13C NMR analysis, we can conclude that the polymer should have -RRRRRRSSSSSS- or -SSSSSSRRRRRR- sequences in the main chain. In fact, the polycarbonates produced from racemic-1 has a stereo multiblock structure with alternating blocks of (R)- and (S)-polymer segments, rather than a stereocomplex of two highly enantiomerically enriched chains. A statistical model was used to simulate the spectrum of the PCHC with stereochemical defects formed in the polymer growth-chain transfer between (R,R,R,R)-1 and (S,S,S,S)-1, suggesting that a block in the stereo multiblock PCHC contain an average of five enantiomerically pure carbonate units.
In the recent contributions, we demonstrated that the enantiopure biphenol-linked dinuclear Co(III) complex 1 was a privileged chiral catalysts for asymmetric copolymerization of CO2 with various meso-epoxides, showing high activity and excellent enantioselectivity27,28,29. The mechanistic study revealed that chain-growth step predominantly involves an intramolecular bimetallic cooperation mechanism, wherein alternating chain growth and dissociation of propagating carboxylate species takes turn between two Co(III) ions from the inside cleft of dinuclear Co(III) catalysts by the nucleophilic attack of the growing carboxylate species at one metal centre towards the activated epoxide at the other44. It was also found that the propagating polymer chain transfer could be caused by protic solvents such as water and methanol, rather than the excess cocatalyst. Although every effort has been made to keep the copolymerization reaction anhydrous, we were concerned that trace quantities of water might be present, and thereby cause the growing polymer-chain transfer. Furthermore, it was found that the addition of 10 equiv. of methanol resulted in the significant decrease in copolymer molecular weight from 32.0 to 10.1 kg mol−1, and a slight loss in Pm from 0.82 to 0.79 (Table 1, entry 8).
On the basis of the evidence and analysis mentioned above, the possible formation process for isotactic multiblock PCHC is proposed in the Fig. 7. Since the insertion of CO2 into the growing polymer chain is a fast process, the predominant ring-opening event is the reactions of (S)-1 with (R)-C−O and (R)-1 with (S)-C−O bond of CHO, affording (S)-PCHC and (R)-PCHC, respectively (enantiomeric active species A and B). However, the adventours water or the addition of protic solvent probably results in polymer-chain exchange between the (R)-PCHC anchored on (R)-1 and the (S)-PCHC anchored on (S)-1, affording the (S)-PCHC anchored on (R)-1 and the (R)-PCHC anchored on (S)-1 (diastereomeric active species C and D). At this point, polymer chain propagation resumes with the favoured stereoisomer, creating a diblock structure. The polymer chain exchange and propagation take place repeatedly to provide the isotactic multiblock polycarbonates.
It is a pity that the isotactic multiblock PCHC with a Pm of 0.82 was amorphous material with a Tg of 125 °C, slightly higher than the atactic PCHC. Indeed, no crystallizability of the isotacticity-enriched PCHC is not strange. In previous contribution, we have demonstrated that only PCHCs with more than 90% isotacticity were crystallizable26. However, we expect with great passion the formation of the intramolecular stereocomplexed PCHC, since a stereocomplex formed by the polymer assembly of optically active PCHCs with opposite configurations was previously confirmed30. In order to validate our supposition, solid state NMR spectroscopy was also employed for studying the difference of the microstructure of various PCHCs. (Supplementary Fig. 5 and Supplementary Table 1). As anticipated, the T1 value of carbonyl region for the stereocomplexed PCHC is up to 271 s, which is 223 s longer than that for the amorphous PCHC. Similarly, the T1 values of methine (250 and 195 s) and methylene carbons (146 and 110 s) for the stereocomplexed PCHC are significantly longer than that for the amorphous PCHC (35 s for methine region, and 23 and 21 s for methylene carbons). However, for the isotactic multiblock PCHC, the T1 values of carbonyl region and methine carbons are 56 and 39 s, respectively, while that of methylene carbons are 26 and 24 s. These values are slightly higher than that for the amorphous state, but significantly lower than that for the stereocomplexed PCHC.
Discussion
In conclusion, novel intramolecular stereocomplexed polycarbonates were synthesized by the stereoselective copolymerization of CO2 and meso-epoxides using racemic bimetallic cobalt catalyst system. Highly enantioselective chain growth in an enantiopure catalyst molecule and the copolymer-chain transfer between different configuration catalyst molecules results in the formation of the isotactic multiblock polycarbonates. Solid state NMR spectroscopy study suggests that the interaction in the carbonyl region is responsible for the strong crystallization capacity and compact package structure in the crystalline polycarbonates. This is the only example for the synthesis of crystalline CO2 polymers from racemic catalyst. Due to the use of the inexpensive racemic or achiral ligand, the present synthesis strategy is of great importance for preparing various intramolecular stereocomplexed polycarbonates with enhanced thermal stability.
Methods
General
All manipulations involving air- and/or water-sensitive compounds were carried out in a glove box or with the standard Schlenk techniques under dry nitrogen. CO2 (99.995%) was purchased from Dalian Institute of Special Gases and used as received. Methylene chloride and chloroform were distilled from calcium hydride under nitrogen. Tetrahydrofuran and toluene were distilled from sodium/benzophenone under nitrogen. Epoxides were purchased from Acros and distilled over calcium hydride.
Fast-scan chip calorimeter
Fast-scan chip calorimetry (FSC) was performed with the commercialized FSC (Flash DSC1, Mettler-Toledo, Switzerland). The empty chip-sensor was calibrated according to the standard procedure before the experiment. The ready temperature of test module for all measurements was set as 30 °C. Purge nitrogen gas was used as the protection atmosphere with the constant flow rate 50 ml min−1. First cycle: from 20 °C to 280 °C at a heating rate of 3,000 K s−1, and holding at 280 °C for 5 min, and from 280 to 20 °C at a cooling rate of 3,000 K s−1. Second cycle: from 20 °C to 400 °C at a heating rate of 3,000 K s−1. For all FSC analysis, the result was given based on second cycle.
Solid state NMR experiments
Solid state NMR experiments were performed using an Agilent DD2-500 MHz NMR spectrometer in 4-mm ZrO2 rotors at MAS frequencies ranging from 12 to 14 kHz. 13C cross-polarisation MAS NMR spectra were collected at 125 MHz with a B1(13C) field nutation frequency of 100 kHz, a contact time of 3 ms and a recycle delay of 4 s. 13C spin-lattice relaxation experiments were carried out under CPMAS conditions using the saturation recovery-based sequence. The chemical shifts were referenced to the adamantane with the upfield methine peak at 29.5 p.p.m.
Details of other experiments see Supplementary Methods.
Additional information
How to cite this article: Liu, Y. et al. Crystalline CO2-based polycarbonates prepared from racemic catalyst through intramolecularly interlocked assembly. Nat. Commun. 6:8594 doi: 10.1038/ncomms9594 (2015).
References
Darensbourg, D. J. Making plastics from carbon dioxide: salen metal complexes as catalysts for the production of polycarbonates from epoxides and CO2 . Chem. Rev. 107, 2388–2410 (2007).
Luinstra, G. A. Poly(propylene carbonate), old copolymers of propylene oxide and carbon dioxide with new interests: catalysis and material properties. Polym. Rev. 48, 192–219 (2008).
Kember, M. R., Buchard, A. & Williams, C. K. Catalysts for CO2/epoxide copolymerization. Chem. Commun. 47, 141–163 (2011).
Klaus, S., Lehenmeier, M. W., Anderson, C. E. & Rieger, B. Recent advances in CO2/epoxide copolymerization-new strategies and cooperative mechanisms. Coord. Chem. Rev. 255, 1460–1479 (2011).
Lu, X. B., Ren, W. M. & Wu, G. P. CO2 copolymers from epoxides: catalyst activity, product selectivity, and stereochemistry control. Acc. Chem. Res. 45, 1721–1735 (2012).
Ian Childers, M., Longo, J. M., Van Zee, N. J., LaPointe, A. M. & Coates, G. W. Stereoselective epoxide polymerization and copolymerization. Chem. Rev. 114, 8129–8152 (2014).
Darensbourg, D. J. et al. Catalytic activity of a series of Zn(II) phenoxides for the copolymerization of epoxides and carbon dioxide. J. Am. Chem. Soc. 121, 107–116 (1999).
Moore, D. R., Cheng, M., Lobkovsky, E. B. & Coates, G. W. Electronic and steric effects on catalysts for CO2/epoxide polymerization: subtle modifications resulting in superior activities. Angew. Chem. Int. Ed. 41, 2599–2602 (2002).
Kember, M. R., Knight, P. D., Reung, P. T. R. & Williams, C. K. Highly active dizinc catalyst for the copolymerization of carbon dioxide and cyclohexene oxide at one atmosphere pressure. Angew. Chem. Int. Ed. 48, 931–933 (2009).
Lehenmeier, M. W. et al. Flexibly tethered dinuclear zinc complexes: a solution to the entropy problem in CO2/epoxide copolymerization catalysis? Angew. Chem. Int. Ed. 52, 9821–9826 (2013).
Ellis, W. C. et al. Copolymerization of CO2 and meso epoxides using enantioselective β-diiminate catalysts: a route to highly isotactic polycarbonates. Chem. Sci. 5, 4004–4011 (2014).
Kissling, S. et al. Dinuclear zinc catalysts with unprecedented activities for the copolymerization of cyclohexene oxide and CO2 . Chem. Commun. 51, 4579–4582 (2015).
Kember, M. R. & Williams, C. K. Efficient magnesium catalysts for the copolymerization of epoxides and CO2; using water to synthesize polycarbonate polyols. J. Am. Chem. Soc. 134, 15676–15679 (2012).
Darensbourg, D. J. & Yarbrough, J. C. Mechanistic aspects of the copolymerization reaction of carbon dioxide and epoxides, using a chiral salen chromium chloride catalyst. J. Am. Chem. Soc. 124, 6335–6342 (2002).
Harrold, N. D., Li, Y. & Chisholm, M. H. Studies of ring-opening reactions of styrene oxide by chromium tetraphenylporphyrin initiators. mechanistic and stereochemical considerations. Macromolecules 46, 692–698 (2013).
Qin, Z. Q., Thomas, C. M., Lee, S. & Coates, G. W. Cobalt-based complexes for the copolymerization of propylene oxide and CO2: active and selective catalysts for polycarbonate synthesis. Angew. Chem. Int. Ed. 42, 5484–5487 (2003).
Lu, X. B. & Wang, Y. Highly active, binary catalyst systems for the alternating copolymerization of CO2 and epoxides under mild conditions. Angew. Chem. Int. Ed. 43, 3574–3577 (2004).
Shi, L. et al. Asymmetric alternating copolymerization and terpolymerization of epoxides with carbon dioxide at mild conditions. Macromolecules 39, 5679–5685 (2006).
Noh, E. K., Na, S. J., Sujith, S., Kim, S. W. & Lee, B. Y. Two components in a molecule: highly efficient and thermally robust catalytic system for CO2/epoxide copolymerization. J. Am. Chem. Soc. 129, 8082–8083 (2007).
Ren, W. M., Liu, Z. W., Wen, Y. Q., Zhang, R. & Lu, X. B. Mechanistic aspects of the copolymerization of CO2 with epoxides using a thermally stable single-site cobalt(III) catalyst. J. Am. Chem. Soc. 131, 11509–11518 (2009).
Nishioka, K., Goto, H. & Sugimoto, H. Dual catalyst system for asymmetric alternating copolymerization of carbon dioxide and cyclohexene oxide with chiral aluminum complexes: Lewis base as catalyst activator and Lewis acid as monomer activator. Macromolecules 45, 8172–8192 (2012).
Coates, G. W. Precise control of polyolefin stereochemistry using single-site metal catalysts. Chem. Rev. 100, 1223–1252 (2000).
Wu, G. P. et al. Crystalline CO2 copolymer from epichlorohydrin via Co(III)-complex-mediated stereospecific polymerization. Macromolecules 46, 2128–2133 (2013).
Ren, W. M., Liang, M. W., Xu, Y. C. & Lu, X. B. Trivalent cobalt complex mediated formation of stereoregular CO2 copolymers from phenyl glycidyl ether. Polym. Chem. 4, 4425–4433 (2013).
Nakano, K., Kobayashi, K., Ohkawara, T., Imoto, H. & Nozaki, K. Copolymerization of epoxides with carbon dioxide catalyzed by iron−corrole complexes: synthesis of a crystalline copolymer. J. Am. Chem. Soc. 135, 8456–8459 (2013).
Wu, G. P. et al. Enhanced asymmetric induction for the copolymerization of CO2 and cyclohexene oxide with unsymmetric enantiopure salenCo(III) complexes: synthesis of crystalline CO2-based polycarbonate. J. Am. Chem. Soc. 134, 5682–5688 (2012).
Liu, Y., Ren, W. M., Liu, J. & Lu, X. B. Asymmetric copolymerization of CO2 with meso-epoxides mediated by dinuclear cobalt(III) complexes: unprecedented enantioselectivity and activity. Angew. Chem. Int. Ed. 52, 11594–11598 (2013).
Liu, Y. et al. Stereospecific CO2 copolymers from 3,5-dioxaepoxides: crystallization and functionallization. Macromolecules 47, 1269–1276 (2014).
Liu, Y., Ren, W. M., He, K. K. & Lu, X. B. Crystalline-gradient polycarbonates prepared from enantioselective terpolymerization of meso-epoxides with CO2 . Nat. Commun. 5, 5687 (2014).
Wu, G. P. et al. Stereoregular poly(cyclohexene carbonate)s: unique crystallization behavior. Chin. J. Polym. Sci. 30, 487–492 (2012).
Auriemma, F. et al. Stereocomplexed poly(limonene carbonate): a unique example of the cocrystallization of amorphous enantiomeric polymers. Angew. Chem. Int. Ed. 54, 1215–1218 (2015).
Liu, Y., Ren, W. M., Wang, M., Liu, C. & Lu, X. B. Crystalline stereocomplexed polycarbonates: hydrogen-bond-driven interlocked orderly assembly of the opposite enantiomers. Angew. Chem. Int. Ed. 54, 2241–2244 (2015).
Ovitt, T. M. & Coates, G. W. Stereochemistry of lactide polymerization with chiral catalysts: new opportunities for stereocontrol using polymer exchange mechanisms. J. Am. Chem. Soc. 124, 1316–1326 (2002).
Hirahata, W., Thomas, R. M., Lobkovsky, E. B. & Coates, G. W. Enantioselective polymerization of epoxides: a highly active and selective catalyst for the preparation of stereoregular polyethers and enantiopure epoxides. J. Am. Chem. Soc. 130, 17658–17659 (2008).
Widger, P. C. B. et al. Isospecific polymerization of racemic epoxides: a catalyst system for the synthesis of highly isotactic polyethers. Chem. Commun. 46, 2935–2937 (2010).
Thomas, R. M. et al. Enantioselective epoxide polymerization using a bimetallic cobalt catalyst. J. Am. Chem. Soc. 132, 16520–16525 (2010).
Gomez, M. A., Tanaka, H. & Tonelli, A. E. High-resolution solid-state 13C nuclear magnetic resonance study of isotactic polypropylene polymorphs. Polymer 28, 2227–2232 (1987).
Lauprete, F. Applications of high-resolution solid-state carbon-13 NMR to polymers. Prog. Polym. Sci. 15, 425–474 (1990).
Hansen, M. R., Graf, R. & Spiess, H. W. Solid-state NMR in macromolecular systems: insights on how molecular entities move. Acc. Chem. Res. 46, 1996–2007 (2013).
Policianová, O., Hodan, J., Brus, J. & Kotek, J. Origin of toughness in β-polypropylene: the effect of molecular mobility in the amorphous phase. Polymer 60, 107–114 (2015).
Nozaki, K., Nakano, K. & Hiyama, T. Optically active polycarbonates: asymmetric alternating copolymerization of cyclohexene oxide and carbon dioxide. J. Am. Chem. Soc. 121, 11008–11009 (1999).
Nakano, K., Nozaki, K. & Hiyama, T. Spectral assignment of poly[cyclohexene oxide-alt-carbon dioxide]. Macromolecules 34, 6325–6332 (2001).
Cohen, C. T., Thomas, C. M., Peretti, K. L., Lobkovsky, E. B. & Coates, G. W. Copolymerization of cyclohexene oxide and carbon dioxide using (salen)Co(III) complexes: synthesis and characterization of syndiotactic poly(cyclohexene carbonate). Dalton Trans. 237–249 (2006).
Liu, Y. et al. Mechanistic understanding of dinuclear cobalt(III) complex mediated highly enantioselective copolymerization of meso-epoxides with CO2 . Macromolecules 47, 7775–7788 (2014).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (NSFC, Grant 21134002, 21373035), and Program for Chang Jiang Scholars and Innovative Research Team in University (IRT13008). X.-B.L. gratefully acknowledges the Chang Jiang Scholars Program (T2011056) from the Ministry of Education of China. We are also grateful to Professor Wen-Bing Hu for his kind assistance in FSC analysis.
Author information
Authors and Affiliations
Contributions
Y.L. performed catalytic experiments, measurements, the copolymer characterization and mechanistic study. W.-M.R. participated in discussions and contributed important suggestions. W.-P.Z. and R.-R.Z. performed the solid state NMR analysis. X.-B.L. designed the research and conducted the experiments. X.-B.L. and Y.L. co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-5, Supplementary Table 1, Supplementary Methods and Supplementary References (PDF 422 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, Y., Ren, WM., Zhang, WP. et al. Crystalline CO2-based polycarbonates prepared from racemic catalyst through intramolecularly interlocked assembly. Nat Commun 6, 8594 (2015). https://doi.org/10.1038/ncomms9594
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms9594
This article is cited by
-
Stereoselective synthesis of biodegradable polymers by salen-type metal catalysts
Science China Chemistry (2022)
-
Fast-scan chip-calorimetry measurement on crystallization and enthalpy relaxation kinetics of isotactic poly(cyclohexene carbonate)
Journal of Polymer Research (2021)
-
Understanding metal synergy in heterodinuclear catalysts for the copolymerization of CO2 and epoxides
Nature Chemistry (2020)
-
Mechanism of Heat-Induced Fusion of Silver Nanowires
Scientific Reports (2020)
-
Stereoselective photoredox ring-opening polymerization of O-carboxyanhydrides
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.