Abstract
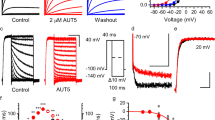
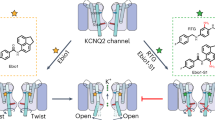
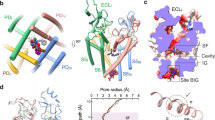
Most known small-molecule inhibitors of voltage-gated ion channels have poor subtype specificity because they interact with a highly conserved binding site in the central cavity. Using alanine-scanning mutagenesis, electrophysiological recordings and molecular modeling, we have identified a new drug-binding site in Kv1.x channels. We report that Psora-4 can discriminate between related Kv channel subtypes because, in addition to binding the central pore cavity, it binds a second, less conserved site located in side pockets formed by the backsides of S5 and S6, the S4-S5 linker, part of the voltage sensor and the pore helix. Simultaneous drug occupation of both binding sites results in an extremely stable nonconducting state that confers high affinity, cooperativity, use-dependence and selectivity to Psora-4 inhibition of Kv1.x channels. This new mechanism of inhibition represents a molecular basis for the development of a new class of allosteric and selective voltage-gated channel inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wulff, H., Castle, N.A. & Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 8, 982–1001 (2009).
Hockerman, G.H., Dilmac, N., Scheuer, T. & Catterall, W.A. Molecular determinants of diltiazem block in domains IIIS6 and IVS6 of L-type Ca2+ channels. Mol. Pharmacol. 58, 1264–1270 (2000).
Payandeh, J., Scheuer, T., Zheng, N. & Catterall, W.A. The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358 (2011).
Ragsdale, D.S., McPhee, J.C., Scheuer, T. & Catterall, W.A. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc. Natl. Acad. Sci. USA 93, 9270–9275 (1996).
Ragsdale, D.S., McPhee, J.C., Scheuer, T. & Catterall, W.A. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science 265, 1724–1728 (1994).
Decher, N. et al. Molecular basis for Kv1.5 channel block: conservation of drug binding sites among voltage-gated K+ channels. J. Biol. Chem. 279, 394–400 (2004).
Mitcheson, J.S., Chen, J., Lin, M., Culberson, C. & Sanguinetti, M.C. A structural basis for drug-induced long QT syndrome. Proc. Natl. Acad. Sci. USA 97, 12329–12333 (2000).
Seebohm, G. et al. Molecular determinants of KCNQ1 channel block by a benzodiazepine. Mol. Pharmacol. 64, 70–77 (2003).
Hanner, M. et al. Binding of correolide to the Kv1.3 potassium channel: characterization of the binding domain by site-directed mutagenesis. Biochemistry 40, 11687–11697 (2001).
MacKinnon, R., Heginbotham, L. & Abramson, T. Mapping the receptor site for charybdotoxin, a pore-blocking potassium channel inhibitor. Neuron 5, 767–771 (1990).
Swartz, K.J. & MacKinnon, R. Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron 18, 675–682 (1997).
Vennekamp, J. et al. Kv1.3-blocking 5-phenylalkoxypsoralens: a new class of immunomodulators. Mol. Pharmacol. 65, 1364–1374 (2004).
Zimin, P.I. et al. Potassium channel block by a tripartite complex of two cationophilic ligands and a potassium ion. Mol. Pharmacol. 78, 588–599 (2010).
Decher, N. et al. Structural determinants of Kvβ1.3-induced channel inactivation: a hairpin modulated by PIP2 . EMBO J. 27, 3164–3174 (2008).
Decher, N., Kumar, P., Gonzalez, T., Renigunta, V. & Sanguinetti, M.C. Structural basis for competition between drug binding and Kvβ1.3 accessory subunit-induced N-type inactivation of Kv1.5 channels. Mol. Pharmacol. 68, 995–1005 (2005).
Ledwell, J.L. & Aldrich, R.W. Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation. J. Gen. Physiol. 113, 389–414 (1999).
Soler-Llavina, G.J., Chang, T.H. & Swartz, K.J. Functional interactions at the interface between voltage-sensing and pore domains in the Shaker Kv channel. Neuron 52, 623–634 (2006).
Armstrong, C.M. & Hille, B. The inner quaternary ammonium ion receptor in potassium channels of the node of Ranvier. J. Gen. Physiol. 59, 388–400 (1972).
Baukrowitz, T. & Yellen, G. Two functionally distinct subsites for the binding of internal blockers to the pore of voltage-activated K+ channels. Proc. Natl. Acad. Sci. USA 93, 13357–13361 (1996).
Huber, I. et al. Conserved Ca2+-antagonist-binding properties and putative folding structure of a recombinant high-affinity dihydropyridine-binding domain. Biochem. J. 347, 829–836 (2000).
Nau, C. & Wang, G.K. Interactions of local anesthetics with voltage-gated Na+ channels. J. Membr. Biol. 201, 1–8 (2004).
Striessnig, J. et al. Structural basis of drug binding to L Ca2+ channels. Trends Pharmacol. Sci. 19, 108–115 (1998).
Šali, A. & Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Boeckmann, B. et al. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 31, 365–370 (2003).
Goodsell, D.S., Morris, G.M. & Olson, A.J. Automated docking of flexible ligands: applications of AutoDock. J. Mol. Recognit. 9, 1–5 (1996).
Åqvist, J. & Luzhkov, V. Ion permeation mechanism of the potassium channel. Nature 404, 881–884 (2000).
Zhou, Y., Morais-Cabral, J.H., Kaufman, A. & MacKinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel–Fab complex at 2.0 Å resolution. Nature 414, 43–48 (2001).
Kleywegt, G.J. & Jones, T.A. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr. D Biol. Crystallogr. 50, 178–185 (1994).
van Aalten, D.M. et al. PRODRG, a program for generating molecular topologies and unique molecular descriptors from coordinates of small molecules. J. Comput. Aided Mol. Des. 10, 255–262 (1996).
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005).
Scott, W.R.P. et al. The GROMOS biomolecular simulation program package. J. Phys. Chem. A 103, 3596–3607 (1999).
Parrinello, M. & Rahman, A. Polymorphic transitions in single-crystals—a new molecular-dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Hermans, J., Berendsen, H.J.C., Vangunsteren, W.F. & Postma, J.P.M. A consistent empirical potential for water-protein interactions. Biopolymers 23, 1513–1518 (1984).
Hess, B., Bekker, H., Berendsen, H.J.C. & Fraaije, J.G.E.M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38, 27–38 (1996).
Stefani, E. & Bezanilla, F. Cut-open oocyte voltage-clamp technique. Methods Enzymol. 293, 300–318 (1998).
Armstrong, C.M. & Bezanilla, F. Inactivation of the sodium channel. II. Gating current experiments. J. Gen. Physiol. 70, 567–590 (1977).
Acknowledgements
This work was supported by Deutsche Forschungsgemeinschaft grant DE1482-3/2 to N.D. and by the P.E. Kempkes Stiftung 01/2011 to S.R. and S.M. M.S.P.S. is supported by the Wellcome Trust. M.C.S. was supported by US National Institutes of Health (NIH)–NIH Heart, Lung, and Blood Institute grant HL055236. S.M. was supported by the Studienstiftung des Deutschen Volkes e.V.
Author information
Authors and Affiliations
Contributions
N.D. conceived the study. S.M. performed the majority of the experiments. P.J.S. and M.S.P.S. performed the ligand dockings and MDSs. M.R., E.N.-A. and T.B. performed the inside-out macropatch clamp experiments. J.L.A. and M.C.S. performed the cut-open oocyte Vaseline gap measurements. S.R. and S.M. acquired funding. N.D., M.C.S., T.B., S.R., K.S. and S.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 1096 kb)
Supplementary Video 1
Linear interpolation between open (KvChim) and closed (MlotiK) Kv1.5 models (MOV 32029 kb)
Rights and permissions
About this article
Cite this article
Marzian, S., Stansfeld, P., Rapedius, M. et al. Side pockets provide the basis for a new mechanism of Kv channel–specific inhibition. Nat Chem Biol 9, 507–513 (2013). https://doi.org/10.1038/nchembio.1271
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1271
This article is cited by
-
Inhibition of NMDA receptors through a membrane-to-channel path
Nature Communications (2022)
-
Inhibition of the hERG potassium channel by phenanthrene: a polycyclic aromatic hydrocarbon pollutant
Cellular and Molecular Life Sciences (2021)
-
New potential binding determinant for hERG channel inhibitors
Scientific Reports (2016)
-
KCNE1 induces fenestration in the Kv7.1/KCNE1 channel complex that allows for highly specific pharmacological targeting
Nature Communications (2016)
-
Alkanols inhibit voltage-gated K+ channels via a distinct gating modifying mechanism that prevents gate opening
Scientific Reports (2015)