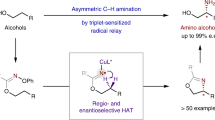
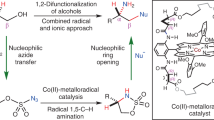
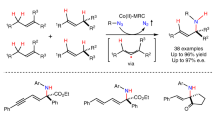
Abstract
A diverse array of chiral organocatalysts have been developed that rely on acid–base interactions to promote enantioselective ionic reactions via the movement of electron pairs. The stereocontrol of radical reactions using organocatalysts is an alternative approach, and several studies have shown that synthetically useful reactivity can result by controlling the movement of single electrons. However, in these studies, it is still an acid–based organocatalyst which forms a closed-shell intermediate with substrate prior to the radical reaction and imparts chiral information, and use of a chiral organic radical directly as catalyst has only rarely been explored. Here, we report the design of an organic thiyl radical catalyst with a carefully designed chiral pocket constructed around a chiral thiol precatalyst. The resulting catalyst was used to effect highly diastereo- and enantioselective C–C bond-forming radical cyclizations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dalko, P. I. Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications (Wiley-VCH, 2013).
Mukherjee, S., Yang, J. W., Hoffmann, S. & List, B. Asymmetric enamine catalysis. Chem. Rev. 107, 5471–5569 (2007).
Lelais, G. & MacMillan, D. W. C. Modern strategies in organic catalysis: the advent and development of iminium activation. Aldrichim. Acta 39, 79–87 (2006).
Akiyama, T. Stronger Brønsted acids. Chem. Rev. 107, 5744–5758 (2007).
Terada, M. Chiral phosphoric acids as versatile catalysts for enantioselective transformations. Synthesis 2010, 1929–1982 (2010).
Taylor, M. S. & Jacobsen, E. N. Asymmetric catalysis by chiral hydrogen-bond donors. Angew. Chem. Int. Ed. 45, 1520–1543 (2006).
Brak, K. & Jacobsen, E. N. Asymmetric ion-pairing catalysis. Angew. Chem. Int. Ed. 52, 534–561 (2013).
Chatgilialoglu, C. & Studer, A. Encyclopedia of Radicals in Chemistry, Biology and Materials (Wiley, 2012).
Frey, P. A., Hegeman, A. D. & Reed, G. H. Free radical mechanisms in enzymology. Chem. Rev. 106, 3302–3316 (2006).
Sibi, M. P., Manyem, S. & Zimmerman, J. Enantioselective radical processes. Chem. Rev. 103, 3263–3296 (2003).
Bauer, A., Westkämper, F., Grimme, S. & Bach, T. Catalytic enantioselective reactions driven by photoinduced electron transfer. Nature 436, 1139–1140 (2005).
Beeson, T. D., Mastracchio, A., Hong, J-B., Ashton, K. & MacMillan, D. W. C. Enantioselective organocatalysis using SOMO activation. Science 316, 582–585 (2007).
Sibi, M. P. & Hasegawa, M. Organocatalysis in radical chemistry. Enantioselective α-oxyamination of aldehydes. J. Am. Chem. Soc. 129, 4124–4125 (2007).
Nicewicz, D. A. & MacMillan, D. W. C. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 322, 77–80 (2008).
Arceo, E., Jurberg, I. D., Álvarez-Fernández, A. & Melchiorre, P. Photochemical activity of a key donor–acceptor complex can drive stereoselective catalytic α-alkylation of aldehydes. Nature Chem. 5, 750–756 (2013).
Rono, L. J., Yayla, H. G., Wang, D. Y., Armstrong, M. F. & Knowles, R. R. Enantioselective photoredox catalysis enabled by proton-coupled electron transfer: development of an asymmetric aza-pinacol cyclization. J. Am. Chem. Soc. 135, 17735–17738 (2013).
Brimioulle, R. & Bach, T. Enantioselective Lewis acid catalysis of intramolecular enone [2+2] photocycloaddition reactions. Science 342, 840–843 (2013).
Dénès, F., Pichowicz, M., Povie, G. & Renaud, P. Thiyl radicals in organic synthesis. Chem. Rev. 114, 2587–2693 (2014).
Roberts, B. P. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 28, 25–35 (1999).
Haque, M. B. & Roberts, B. P. Enantioselective radical-chain hydrosilylation of prochiral alkenes using optically active thiol catalysts. Tetrahedron Lett. 37, 9123–9126 (1996).
Cai, Y., Roberts, B. P. & Tocher, D. A. Carbohydrate-derived thiols as protic polarity-reversal catalysts for enantioselective radical-chain reactions. J. Chem. Soc. Perkin Trans. 1, 1376–1386 (2002).
Qiao, C. & Marsh, E. N. G. Mechanism of benzylsuccinate synthase: stereochemistry of toluene addition to fumarate and maleate. J. Am. Chem. Soc. 127, 8608–8609 (2005).
Miura, K., Fugami, K., Oshima, K. & Utimoto, K. Synthesis of vinylcyclopentanes from vinylcyclopropanes and alkenes promoted by benzenethiyl radical. Tetrahedron Lett. 29, 5135–5138 (1988).
Feldman, K. S., Romanelli, A. L., Ruckle, R. E. & Miller, R. F. Cyclopentane synthesis via free radical mediated addition of functionalized alkenes to substituted vinyl cyclopropanes. J. Am. Chem. Soc. 110, 3300–3302 (1988).
Hancock, A. N. & Schiesser, C. H. Guidelines for radical reactions: some thirty years on. Chem. Commun. 49, 9892–9895 (2013).
Jiao, L. & Yu, Z-X. Vinylcyclopropane derivatives in transition-metal-catalyzed cycloadditions for the synthesis of carbocyclic compounds. J. Org. Chem. 78, 6842–6848 (2013).
Xu, H., Qu, J-P., Liao, S., Xiong, H. & Tang, Y. Highly enantioselective [3+2] annulation of cyclic enol silyl ethers with donor–acceptor cyclopropanes: accessing 3a-hydroxy [n.3.0]carbobicycles. Angew. Chem. Int. Ed. 52, 4004–4007 (2013).
Giacalone, F., Gruttadauria, M., Agrigento, P. & Noto, R. Low-loading asymmetric organocatalysis. Chem. Soc. Rev. 41, 2406–2447 (2012).
Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research from the MEXT (Japan). Y.K. acknowledges a Grant-in-Aid for the Research Fellowship of JSPS for Young Scientists.
Author information
Authors and Affiliations
Contributions
T.H. conceived the study and wrote the manuscript. T.H. and Y.K. designed experiments and Y.K. performed experiments. K.M. organized the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5067 kb)
Supplementary information
Crystallographic data for compound 2a' (CIF 30 kb)
Supplementary information
Crystallographic data for compound (S)-5b (CIF 50 kb)
Rights and permissions
About this article
Cite this article
Hashimoto, T., Kawamata, Y. & Maruoka, K. An organic thiyl radical catalyst for enantioselective cyclization. Nature Chem 6, 702–705 (2014). https://doi.org/10.1038/nchem.1998
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1998
This article is cited by
-
A general copper-catalysed enantioconvergent radical Michaelis–Becker-type C(sp3)–P cross-coupling
Nature Synthesis (2023)
-
Visible-light mediated catalytic asymmetric radical deuteration at non-benzylic positions
Nature Communications (2022)
-
Catalytic enantioselective desymmetrizing functionalization of alkyl radicals via Cu(i)/CPA cooperative catalysis
Nature Catalysis (2020)
-
Iron-catalysed asymmetric carboazidation of styrenes
Nature Catalysis (2020)
-
Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates
Nature Chemistry (2020)