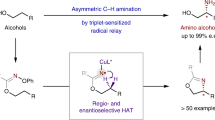
Abstract
Chiral amines are among the most important organic compounds and have widespread applications. Enantioselective construction of chiral amines is a major aim in organic synthesis. Among synthetic methods, direct functionalization of omnipresent C–H bonds with common organic nitrogen compounds represents one of the most attractive strategies. However, C–H amination strategies are largely limited to constructing a specific type of N-heterocycles or amine derivatives. To maximize the synthetic potential of asymmetric C–H amination, we report here an approach that unites the complementary reactivities of radical and ionic chemistry for streamlined synthesis of functionalized chiral amines. This synthesis merges the development of an enantioselective radical process for 1,5-C(sp3)–H amination of alkoxysulfonyl azides via Co(II)-based metalloradical catalysis with an enantiospecific ionic process for ring-opening of the resulting five-membered chiral sulfamidates by nucleophiles. Given that alkoxysulfonyl azides are derived from the corresponding alcohols, this approach offers a powerful synthetic tool for enantioselective β-C–H amination of common alcohols while converting the hydroxy group to other functionalities through formal nucleophilic substitution.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2097080 (2l), 2097081 (2t), 2097082 (2ae), 2097083 (2e), 2097084 (2a), 2097085 (3m), 2097086 (3v), 2097087 (3i), 2097088 (N-Bn-2ad), 2097089 (2u), 2097090 (2r) and 2097091 (2c). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data that support the findings of this study, which include experimental procedures and compound characterization, are available within the paper and its Supplementary Information.
References
Renaud, P. & Sibi, M. P. Radicals in Organic Synthesis (Wiley-VCH, 2001).
Zard, S. Z. Radical Reactions in Organic Synthesis (Oxford Univ. Press, 2003).
Curran, D. P., Porter, N. A. & Giese, B. Stereochemistry of Radical Reactions: Concepts, Guidelines, and Synthetic Applications (John Wiley & Sons, 2008).
Sibi, M. P., Manyem, S. & Zimmerman, J. Enantioselective radical processes. Chem. Rev. 103, 3263–3296 (2003).
Kern, N., Plesniak, M. P., McDouall, J. J. W. & Procter, D. J. Enantioselective cyclizations and cyclization cascades of samarium ketyl radicals. Nat. Chem. 9, 1198–1204 (2017).
Li, J. et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature 574, 516–521 (2019).
Wang, F., Chen, P. & Liu, G. Copper-catalyzed radical relay for asymmetric radical transformations. Acc. Chem. Res. 51, 2036–2046 (2018).
Dong, X.-Y. et al. A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling. Nat. Chem. 11, 1158–1166 (2019).
Gu, Q.-S., Li, Z.-L. & Liu, X.-Y. Copper(I)-catalyzed asymmetric reactions involving radicals. Acc. Chem. Res. 53, 170–181 (2020).
Demarteau, J., Debuigne, A. & Detrembleur, C. Organocobalt complexes as sources of carbon-centered radicals for organic and polymer chemistries. Chem. Rev. 119, 6906–6955 (2019).
Nugent, W. A. & RajanBabu, T. V. Transition-metal-centered radicals in organic synthesis. Titanium(III)-induced cyclization of epoxy olefins. J. Am. Chem. Soc. 110, 8561–8562 (1988).
Gansäuer, A. et al. Catalysis via homolytic substitutions with C−O and Ti−O bonds: oxidative additions and reductive eliminations in single electron steps. J. Am. Chem. Soc. 131, 16989–16999 (2009).
Yao, C., Dahmen, T., Gansäuer, A. & Norton, J. Anti-Markovnikov alcohols via epoxide hydrogenation through cooperative catalysis. Science 364, 764–767 (2019).
Ye, K.-Y., McCallum, T. & Lin, S. Bimetallic radical redox-relay catalysis for the isomerization of epoxides to allylic alcohols. J. Am. Chem. Soc. 141, 9548–9554 (2019).
Das, S. K., Roy, S., Khatua, H. & Chattopadhyay, B. Iron-catalyzed amination of strong aliphatic C(sp3)–H bonds. J. Am. Chem. Soc. 142, 16211–16217 (2020).
Goswami, M. et al. Characterization of porphyrin-Co(III)-‘nitrene radical’ species relevant in catalytic nitrene transfer reactions. J. Am. Chem. Soc. 137, 5468–5479 (2015).
Jiang, H., Lang, K., Lu, H., Wojtas, L. & Zhang, X. P. Asymmetric radical bicyclization of allyl azidoformates via cobalt(II)-based metalloradical catalysis. J. Am. Chem. Soc. 139, 9164–9167 (2017).
Li, C. Q. et al. Catalytic radical process for enantioselective amination of C(sp3)–H bonds. Angew. Chem. Int. Ed. 57, 16837–16841 (2018).
Bower, J. F., Rujirawanich, J. & Gallagher, T. N-Heterocycle construction via cyclic sulfamidates. Applications in synthesis. Org. Biomol. Chem. 8, 1505–1519 (2010).
Meléndez, R. E. & Lubell, W. D. Synthesis and reactivity of cyclic sulfamidites and sulfamidates. Tetrahedron 59, 2581–2616 (2003).
Yin, Q., Shi, Y., Wang, J. & Zhang, X. Direct catalytic asymmetric synthesis of α-chiral primary amines. Chem. Soc. Rev. 49, 6141–6153 (2020).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev. 117, 9247–9301 (2017).
Milczek, E., Boudet, N. & Blakey, S. Enantioselective C–H amination using cationic ruthenium(II)–pybox catalysts. Angew. Chem. Int. Ed. 47, 6825–6828 (2008).
Zalatan, D. N. & Du Bois, J. A chiral rhodium carboxamidate catalyst for enantioselective C−H amination. J. Am. Chem. Soc. 130, 9220–9221 (2008).
Adams, C. S., Boralsky, L. A., Guzei, I. A. & Schomaker, J. M. Modular functionalization of allenes to aminated stereotriads. J. Am. Chem. Soc. 134, 10807–10810 (2012).
Alderson, J. M., Phelps, A. M., Scamp, R. J., Dolan, N. S. & Schomaker, J. M. Ligand-controlled, tunable silver-catalyzed C–H amination. J. Am. Chem. Soc. 136, 16720–16723 (2014).
Adams, C. S., Grigg, R. D. & Schomaker, J. M. Complete stereodivergence in the synthesis of 2-amino-1,3-diols from allenes. Chem. Sci. 5, 3046–3056 (2014).
Miyazawa, T. et al. Chiral paddle-wheel diruthenium complexes for asymmetric catalysis. Nat. Catal. 3, 851–858 (2020).
Nakafuku, K. M. et al. Enantioselective radical C–H amination for the synthesis of β-amino alcohols. Nat. Chem. 12, 697–704 (2020).
Roizen, J. L., Harvey, M. E. & Du Bois, J. Metal-catalyzed nitrogen-atom transfer methods for the oxidation of aliphatic C–H bonds. Acc. Chem. Res. 45, 911–922 (2012).
Thornton, A. R., Martin, V. I. & Blakey, S. B. π-Nucleophile traps for metallonitrene/alkyne cascade reactions: a versatile process for the synthesis of α-aminocyclopropanes and β-aminostyrenes. J. Am. Chem. Soc. 131, 2434–2435 (2009).
Burke, E. G. & Schomaker, J. M. Oxidative allene amination for the synthesis of azetidin-3-ones. Angew. Chem. Int. Ed. 54, 12097–12101 (2015).
Liang, J.-L., Yuan, S.-X., Huang, J.-S., Yu, W.-Y. & Che, C.-M. Highly diastereo- and enantioselective intramolecular amidation of saturated C–H bonds catalyzed by ruthenium porphyrins. Angew. Chem. Int. Ed. 41, 3465–3468 (2002).
Hu, Y. et al. Next-generation D2-symmetric chiral porphyrins for cobalt(II)-based metalloradical catalysis: catalyst engineering by distal bridging. Angew. Chem. Int. Ed. 58, 2670–2674 (2019).
Ruppel, J. V. et al. A highly effective cobalt catalyst for olefin aziridination with azides: hydrogen bonding guided catalyst design. Org. Lett. 10, 1995–1998 (2008).
Chen, Y., Fields, K. B. & Zhang, X. P. Bromoporphyrins as versatile synthons for modular construction of chiral porphyrins: cobalt-catalyzed highly enantioselective and diastereoselective cyclopropanation. J. Am. Chem. Soc. 126, 14718–14719 (2004).
Xu, X. et al. Highly asymmetric intramolecular cyclopropanation of acceptor-substituted diazoacetates by Co(II)-based metalloradical catalysis: iterative approach for development of new-generation catalysts. J. Am. Chem. Soc. 133, 15292–15295 (2011).
Tian, Z., Fattahi, A., Lis, L. & Kass, S. R. Cycloalkane and cycloalkene C–H bond dissociation energies. J. Am. Chem. Soc. 128, 17087–17092 (2006).
Rousseau, J.-F., Chekroun, I., Ferey, V. & Labrosse, J. R. Concise preparation of a stable cyclic sulfamidate intermediate in the synthesis of a enantiopure chiral active diamine derivative. Org. Process Res. Dev. 19, 506–513 (2015).
Diosdado, S., López, R. & Palomo, C. Ureidopeptide-based Brønsted bases: design, synthesis and application to the catalytic enantioselective synthesis of β-amino nitriles from (arylsulfonyl)acetonitriles. Chem. Eur. J. 20, 6526–6531 (2014).
Kaseda, T., Kikuchi, T. & Kibayashi, C. Enantioselective total synthesis of (+)-(S)-dihydroperiphylline. Tetrahedron Lett. 30, 4539–4542 (1989).
Wolfard, J., Xu, J., Zhang, H. & Chung, C. K. Synthesis of chiral tryptamines via a regioselective indole alkylation. Org. Lett. 20, 5431–5434 (2018).
Lucet, D., Le Gall, T. & Mioskowski, C. The chemistry of vicinal diamines. Angew. Chem. Int. Ed. 37, 2580–2627 (1998).
Rathi, A. K., Syed, R., Shin, H.-S. & Patel, R. V. Piperazine derivatives for therapeutic use: a patent review (2010–present). Expert Opin. Ther. Pat. 26, 777–797 (2016).
Tasso, B. et al. Synthesis, binding, and modeling studies of new cytisine derivatives, as ligands for neuronal nicotinic acetylcholine receptor subtypes. J. Med. Chem. 52, 4345–4357 (2009).
Vara, B. A. & Johnston, J. N. Enantioselective synthesis of β-fluoro amines via β-amino α-fluoro nitroalkanes and a traceless activating group strategy. J. Am. Chem. Soc. 138, 13794–13797 (2016).
Ishizuka, T., Ishibuchi, S. & Kunieda, T. Chiral synthons for 2-amino alcohols. Facile preparation of optically active amino hydroxy acids of biological interest. Tetrahedron 49, 1841–1852 (1993).
Muñiz, K. Imido-osmium(VIII) compounds in organic synthesis: aminohydroxylation and diamination reactions. Chem. Soc. Rev. 33, 166–174 (2004).
Cardona, F. & Goti, A. Metal-catalysed 1,2-diamination reactions. Nat. Chem. 1, 269–275 (2009).
Pendem, N. et al. Helix-forming propensity of aliphatic urea oligomers incorporating noncanonical residue substitution patterns. J. Am. Chem. Soc. 135, 4884–4892 (2013).
Jiang, H., Lang, K., Lu, H., Wojtas, L. & Zhang, X. P. Intramolecular radical aziridination of allylic sulfamoyl azides by cobalt(II)-based metalloradical catalysis: effective construction of strained heterobicyclic structures. Angew. Chem. Int. Ed. 55, 11604–11608 (2016).
Reitz, A. B., Smith, G. R. & Parker, M. H. The role of sulfamide derivatives in medicinal chemistry: a patent review (2006–2008). Expert Opin. Ther. Pat. 19, 1449–1453 (2009).
Cavender, C. J. & Shiner, V. J. Trifluoromethanesulfonyl azide. Its reaction with alkyl amines to form alkyl azides. J. Org. Chem. 37, 3567–3569 (1972).
Meng, G. et al. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 574, 86–89 (2019).
Acknowledgements
We are grateful for financial support by the NIH (R01-GM132471) (X.P.Z.) and in part by the NSF (CHE-1900375) (X.P.Z.).
Author information
Authors and Affiliations
Contributions
K.L. and Y.H. conducted the experiments. Y.H. initiated the project. K.L. completed the project. W.C.C.L. assisted the project. X.P.Z conceived the work and directed the project. K.L., Y.H. and X.P.Z. designed the experiments. K.L. and X.P.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Bas de Bruin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Discussion and Table 1.
Supplementary Data 1
Crystallographic data for compound 2a, CCDC 2097084.
Supplementary Data 2
Crystallographic data for compound 2ae, CCDC 2097082.
Supplementary Data 3
Crystallographic data for compound 2c, CCDC 2097091.
Supplementary Data 4
Crystallographic data for compound 2e, CCDC 2097083.
Supplementary Data 5
Crystallographic data for compound 2l, CCDC 2097080.
Supplementary Data 6
Crystallographic data for compound 2r, CCDC 2097090.
Supplementary Data 7
Crystallographic data for compound 2t, CCDC 2097081.
Supplementary Data 8
Crystallographic data for compound 2u, CCDC 2097089.
Supplementary Data 9
Crystallographic data for compound 3i, CCDC 2097087.
Supplementary Data 10
Crystallographic data for compound 3m, CCDC 2097085.
Supplementary Data 11
Crystallographic data for compound 3v, CCDC 2097086.
Supplementary Data 12
Crystallographic data for compound NBn2ad, CCDC 2097088.
Rights and permissions
About this article
Cite this article
Lang, K., Hu, Y., Lee, WC.C. et al. Combined radical and ionic approach for the enantioselective synthesis of β-functionalized amines from alcohols. Nat. Synth 1, 548–557 (2022). https://doi.org/10.1038/s44160-022-00107-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00107-3
This article is cited by
-
Stereoselective construction of β-, γ- and δ-lactam rings via enzymatic C–H amidation
Nature Catalysis (2023)
-
Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism
Nature Chemistry (2023)
-
Biomimetic asymmetric catalysis
Science China Chemistry (2023)
-
Chiral amine synthesis refashioned
Nature Synthesis (2022)