Abstract
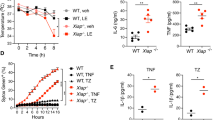
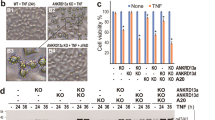
The retinoblastoma tumour suppressor protein RB is cleaved by caspases during apoptosis. Here we have mutated the caspase cleavage site in the carboxy terminus of the murine Rb protein in the mouse germ line to create the Rb-MI allele. After endotoxic shock, expression of Rb-MI inhibits apoptosis in the intestines, but not in the spleen, and promotes the survival of male mice. Fibroblasts expressing Rb-MI protein are protected from apoptosis induced by the tumour-necrosis factor-α type I receptor (TNFRI) but remain sensitive to cell death induced by DNA damage. Correspondingly, the release of cytochrome c and the activation of caspase-3 induced by TNFRI, but not by DNA damage, are defective in cells expressing Rb-MI. Our results highlight the importance of Rb cleavage in TNFRI-induced apoptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cryns, V. & Yuan, J. Proteases to die for. Genes Dev. 12, 1551–1570 (1998).
Budihardjo, I., Oliver, H., Lutter, M., Luo, X. & Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell. Dev. Biol. 15, 269–290 (1999).
Wolf, B. B. & Green, D. R. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J. Biol. Chem. 274, 20049–20052 (1999).
Clem, R. J. et al. c-IAP1 is cleaved by caspases to produce a proapoptotic C-terminal fragment. J. Biol. Chem. 276, 7602–7608 (2001).
Clem, R. J. et al. Modulation of cell death by Bcl-XL through caspase interaction. Proc. Natl Acad. Sci. USA 95, 554–559 (1998).
Luo, X., Budihardjo, I., Zou, H., Slaughter, C. & Wang, X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94, 481–490 (1998).
Li, H., Zhu, H., Xu, C. J. & Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94, 491–501 (1998).
Srinivasula, S. M. et al. The Ced-3/interleukin 1β converting enzyme-like homolog Mch6 and the lamin-cleaving enzyme Mch2α are substrates for the apoptotic mediator CPP32. J. Biol. Chem. 271, 27099–27106 (1996).
Janicke, R. U., Ng, P., Sprengart, M. L. & Porter, A. G. Caspase-3 is required for α-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J. Biol. Chem. 273, 15540–15545 (1998).
Janicke, R. U., Walker, P. A., Lin, X. Y. & Porter, A. G. Specific cleavage of the retinoblastoma protein by an ICE-like protease in apoptosis. EMBO J. 15, 6969–6978 (1996).
Tan, X., Martin, S. J., Green, D. R. & Wang, J. Y. Degradation of retinoblastoma protein in tumor necrosis factor- and CD95-induced cell death. J. Biol. Chem. 272, 9613–9616 (1997).
An, B. & Dou, Q. P. Cleavage of retinoblastoma protein during apoptosis: an interleukin 1 beta-converting enzyme-like protease as candidate. Cancer Res. 56, 438–442 (1996).
Harbour, J. W. & Dean, D. C. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14, 2393–2409 (2000).
Jacks, T. et al. Effects of an Rb mutation in the mouse. Nature 359, 295–300 (1992).
Clarke, A. R. et al. Requirement for a functional Rb-1 gene in murine development. Nature 359, 328–330 (1992).
Lee, E. Y. et al. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359, 288–294 (1992).
Almasan, A. et al. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc. Natl Acad. Sci. USA 92, 5436–5440 (1995).
Phillips, A. C., Ernst, M. K., Bates, S., Rice, N. R. & Vousden, K. H. E2F-1 potentiates cell death by blocking antiapoptotic signaling pathways. Mol. Cell 4, 771–781 (1999).
Tan, X. & Wang, J. Y. The caspase–Rb connection in cell death. Trends Cell Biol. 8, 116–120 (1998).
Boutillier, A. L., Trinh, E. & Loeffler, J. P. Caspase-dependent cleavage of the retinoblastoma protein is an early step in neuronal apoptosis. Oncogene 19, 2171–2178 (2000).
Deng, C., Zhang, P., Harper, J. W., Elledge, S. J. & Leder, P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82, 675–684 (1995).
Hu, N. et al. Heterozygous Rb-1Δ20/+ mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene 9, 1021–1027 (1994).
Pfeffer, K. et al. Mice deficient for the 55 kD tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73, 457–467 (1993).
Piguet, P. F., Vesin, C., Guo, J., Donati, Y. & Barazzone, C. TNF-induced enterocytes apoptosis in mice is mediated by TNF receptor I and does not require p53. Eur. J. Immunol. 28, 3499–3505 (1998).
Borges, H. L. & Linden, R. Gamma irradiation leads to two waves of apoptosis in distinct cell populations of the retina of newborn rats. J. Cell Sci. 112, 4315–4324 (1999).
Rehen, S. K., Varella, M. H., Freitas, F. G., Moraes, M. O. & Linden, R. Contrasting effects of protein synthesis inhibition and of cyclic AMP on apoptosis in the developing retina. Development 122, 1439–1448 (1996).
Lewis, M. et al. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc. Natl Acad. Sci. USA 88, 2830–2834 (1991).
Acehan, D. et al. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell 9, 423–432 (2002).
Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922–2933 (2001).
Du, C., Fang, M., Li, Y., Li, L. & Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 (2000).
Martins, L. M. et al. The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a reaper-like motif. J. Biol. Chem. 277, 439–444 (2002).
Hegde, R. et al. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein–caspase interaction. J. Biol. Chem. 277, 432–438 (2002).
Zou, H., Li, Y., Liu, X. & Wang, X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274, 11549–11556 (1999).
Goldstein, J. C., Waterhouse, N. J., Juin, P., Evan, G. I. & Green, D. R. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nature Cell Biol. 2, 156–162 (2000).
Wallach, D. et al. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17, 331–367 (1999).
Li, K. et al. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 101, 389–399 (2000).
Erickson, S. L. et al. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature 372, 560–563 (1994).
Jupp, O. J. et al. Type II tumour necrosis factor-α receptor (TNFR2) activates c-Jun N- terminal kinase (JNK) but not mitogen-activated protein kinase (MAPK) or p38 MAPK pathways. Biochem. J. 359, 525–535. (2001).
Macleod, K. F., Hu, Y. & Jacks, T. Loss of Rb activates both p53-dependent and independent cell death pathways in the developing mouse nervous system. EMBO J. 15, 6178–6188 (1996).
Tsai, K. Y. et al. Mutation of E2f-1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol. Cell 2, 293–304 (1998).
Moroni, M. C. et al. Apaf-1 is a transcriptional target for E2F and p53. Nature Cell Biol. 3, 552–558 (2001).
Muller, H. et al. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 15, 267–285 (2001).
Guo, Z., Yikang, S., Yoshida, H., Mak, T. W. & Zacksenhaus, E. Inactivation of the retinoblastoma tumor suppressor induces apoptosis protease-activating factor-1 dependent and independent apoptotic pathways during embryogenesis. Cancer Res. 61, 8395–8400 (2001).
Simpson, M. T. et al. Caspase 3 deficiency rescues peripheral nervous system defect in retinoblastoma nullizygous mice. J. Neurosci. 21, 7089–7098 (2001).
Morris, E. J. & Dyson, N. J. Retinoblastoma protein partners. Adv. Cancer Res. 82, 1–54 (2001).
Acknowledgements
We thank members of the Wang lab for comments; M. Kingsbury for help in statistical analysis; R. Johnson for help with ES cell culture; N. Varki for histological analyses; S. Rossi for quantitative cytokine assays and M. Karin for Tnfr1−/- cells. B.N.C. is supported by a Damon Runyon Cancer Research Foundation Fellowship, H.L.B. is supported by a CAPES fellowship (Brazil), and A.M. is supported by the Ernst Schering Research Foundation (Germany). This work was supported by an NIH grant to J.Y.J.W.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chau, B., Borges, H., Chen, TT. et al. Signal-dependent protection from apoptosis in mice expressing caspase-resistant Rb. Nat Cell Biol 4, 757–765 (2002). https://doi.org/10.1038/ncb853
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb853
This article is cited by
-
The proteolytic landscape of cells exposed to non-lethal stresses is shaped by executioner caspases
Cell Death Discovery (2021)
-
The E2F-1 associated retinoblastoma-susceptibility gene product is modified by O-GlcNAc
Amino Acids (2011)
-
Retinoblastoma protein: a central processing unit
Journal of Biosciences (2009)
-
Caspase substrates
Cell Death & Differentiation (2007)
-
Rb plays a role in survival of Abl-dependent human tumor cells as a downstream effector of Abl tyrosine kinase
Oncogene (2006)