Abstract
Induced pluripotent stem cells (iPSCs), which are used to produce transplantable tissues, may particularly benefit older patients, who are more likely to suffer from degenerative diseases. However, iPSCs generated from aged donors (A-iPSCs) exhibit higher genomic instability, defects in apoptosis and a blunted DNA damage response compared with iPSCs generated from younger donors. We demonstrated that A-iPSCs exhibit excessive glutathione-mediated reactive oxygen species (ROS) scavenging activity, which blocks the DNA damage response and apoptosis and permits survival of cells with genomic instability. We found that the pluripotency factor ZSCAN10 is poorly expressed in A-iPSCs and addition of ZSCAN10 to the four Yamanaka factors (OCT4, SOX2, KLF4 and c-MYC) during A-iPSC reprogramming normalizes ROS–glutathione homeostasis and the DNA damage response, and recovers genomic stability. Correcting the genomic instability of A-iPSCs will ultimately enhance our ability to produce histocompatible functional tissues from older patients’ own cells that are safe for transplantation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Prigione, A. et al. Mitochondrial-associated cell death mechanisms are reset to an embryonic-like state in aged donor-derived iPS cells harboring chromosomal aberrations. PLoS ONE 6, e27352 (2011).
Kang, E. et al. Age-related accumulation of somatic mitochondrial DNA mutations in adult-derived human iPSCs. Cell Stem Cell 18, 625–636 (2016).
Lo Sardo, V. et al. Influence of donor age on induced pluripotent stem cells. Nat. Biotechnol. 35, 69–74 (2016).
Garber, K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat. Biotechnol. 33, 890–891 (2015).
Coghlan, A. Unexpected mutations put stem cell trial on hold. New Sci. 227, 9 (2015).
Kang, E. et al. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature 540, 270–275 (2016).
Mandai, M. et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. New Engl. J. Med. 376, 1038–1046 (2017).
Holmstrom, K. M. & Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 15, 411–421 (2014).
Bigarella, C. L., Liang, R. & Ghaffari, S. Stem cells and the impact of ROS signaling. Development 141, 4206–4218 (2014).
Liochev, S. I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 60, 1–4 (2013).
Baccelli, I. & Trumpp, A. The evolving concept of cancer and metastasis stem cells. J. Cell Biol. 198, 281–293 (2012).
Alfadda, A. A. & Sallam, R. M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 936486 (2012).
Sleigh, M. J. The mechanism of DNA breakage by phleomycin in vitro. Nucl. Acids Res. 3, 891–901 (1976).
Franco, R. & Cidlowski, J. A. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 16, 1303–1314 (2009).
Harris, I. S. et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27, 211–222 (2015).
Guo, Z., Kozlov, S., Lavin, M. F., Person, M. D. & Paull, T. T. ATM activation by oxidative stress. Science 330, 517–521 (2010).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Smith, K. P., Luong, M. X. & Stein, G. S. Pluripotency: toward a gold standard for human ES and iPS cells. J. Cell Physiol. 220, 21–29 (2009).
Kim, K. et al. Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 (2010).
Liu, J. C., Lerou, P. H. & Lahav, G. Stem cells: balancing resistance and sensitivity to DNA damage. Trends Cell Biol. 24, 268–274 (2014).
Liu, J. C. et al. High mitochondrial priming sensitizes hESCs to DNA-damage-induced apoptosis. Cell Stem Cell 13, 483–491 (2013).
Zhivotovsky, B. & Kroemer, G. Apoptosis and genomic instability. Nat. Rev. Mol. Cell Biol. 5, 752–762 (2004).
Gurdon, J. B. & Wilmut, I. Nuclear transfer to eggs and oocytes. Cold Spring Harb. Perspect. Biol. 3, 1–14 (2011).
Ma, H. et al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature 511, 177–183 (2014).
Cowan, C. A., Atienza, J., Melton, D. A. & Eggan, K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309, 1369–1373 (2005).
Kim, J., Chu, J., Shen, X., Wang, J. & Orkin, S. H. An extended transcriptional network for pluripotency of embryonic stem cells. Cell 132, 1049–1061 (2008).
Yu, H. B., Kunarso, G., Hong, F. H. & Stanton, L. W. Zfp206, Oct4, and Sox2 are integrated components of a transcriptional regulatory network in embryonic stem cells. J. Biol. Chem. 284, 31327–31335 (2009).
Zhang, W. et al. Zfp206 regulates ES cell gene expression and differentiation. Nucl. Acids Res. 34, 4780–4790 (2006).
Ford, J., Ahmed, S., Allison, S., Jiang, M. & Milner, J. JNK2-dependent regulation of SIRT1 protein stability. Cell Cycle 7, 3091–3097 (2008).
Jiang, J. et al. Zscan4 promotes genomic stability during reprogramming and dramatically improves the quality of iPS cells as demonstrated by tetraploid complementation. Cell Res. 23, 92–106 (2013).
Chan, E. M. et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat. Biotechnol. 27, 1033–1037 (2009).
Tsuda, H., Sasaki, K. & Tanaka, N. Establishment of hypoxanthine phosphoribosyltransferase(HPRT)-locus mutation assay system in mouse ES cells. Altern. Anim. Test. Exp. 11, 118–128 (2005).
Balendiran, G. K., Dabur, R. & Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 22, 343–352 (2004).
Estrela, J. M., Ortega, A. & Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 43, 143–181 (2006).
Traverso, N. et al. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 972913 (2013).
Miller, J. D. et al. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell 13, 691–705 (2013).
Kyttala, A. et al. Genetic variability overrides the impact of parental cell type and determines iPSC differentiation potential. Stem Cell Rep. 6, 200–212 (2016).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Nakagawa, M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26, 101–106 (2008).
Araki, R. et al. Crucial role of c-Myc in the generation of induced pluripotent stem cells. Stem Cells 29, 1362–1370 (2011).
Okita, K. et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 8, 409–412 (2011).
Unternaehrer, J. J. et al. The epithelial-mesenchymal transition factor SNAIL paradoxically enhances reprogramming. Stem Cell Rep. 3, 691–698 (2014).
Baslan, T. et al. Optimizing sparse sequencing of single cells for highly multiplex copy number profiling. Genome Res. 25, 714–724 (2015).
Yamamoto, S. et al. Rapid selection of XO embryonic stem cells using Y chromosome-linked GFP transgenic mice. Transgenic Res. 23, 757–765 (2014).
Seol, H. W. et al. Separation and maintenance of normal cells from human embryonic stem cells with trisomy 12 mosaicism. Chromosome Res. 16, 1075–1084 (2008).
Kilpinen, H. et al. Common genetic variation drives molecular heterogeneity in human iPSCs. Nature 546, 370–375 (2017).
Finkel, T. & Holbrook, N. J. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 (2000).
Shaw, P. X., Werstuck, G. & Chen, Y. Oxidative stress and aging diseases. Oxid. Med. Cell. Longev. 2014, 569146 (2014).
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013).
Hollander, M. & Wolfe, D. A. Nonparametric Statistical Methods (Wiley, 1973).
Kim, K. et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat. Biotechnol. 29, 1117–1119 (2011).
Johnson, G. E. Mammalian cell HPRT gene mutation assay: test methods. Methods Mol. Biol. 817, 55–67 (2012).
Rass, E., Chandramouly, G., Zha, S., Alt, F. W. & Xie, A. Ataxia telangiectasia mutated (ATM) is dispensable for endonuclease I-SceI-induced homologous recombination in mouse embryonic stem cells. J. Biol. Chem. 288, 7086–7095 (2013).
Hahn, P., Kapp, L. N. & Painter, R. B. Establishment and characterization of two human cell lines with amplified dihydrofolate reductase genes. Exp. Cell Res. 168, 89–94 (1987).
Tian, B., Yang, J. & Brasier, A. R. Two-step cross-linking for analysis of protein–chromatin interactions. Methods Mol. Biol. 809, 105–120 (2012).
Buzzard, J. J., Gough, N. M., Crook, J. M. & Colman, A. Karyotype of human ES cells during extended culture. Nat. Biotechnol. 22, 381–382 (2004).
Imreh, M. P. et al. In vitro culture conditions favoring selection of chromosomal abnormalities in human ES cells. J. Cell. Biochem. 99, 508–516 (2006).
Li, S. S. et al. Characterization and gene expression profiling of five new human embryonic stem cell lines derived in Taiwan. Stem Cells Dev. 15, 532–555 (2006).
Plaia, T. W. et al. Characterization of a new NIH-registered variant human embryonic stem cell line, BG01V: a tool for human embryonic stem cell research. Stem Cells 24, 531–546 (2006).
Altmann, H. M. et al. Homozygous/compound heterozygous triadin mutations associated with autosomal-recessive long-QT syndrome and pediatric sudden cardiac arrest: elucidation of the triadin knockout syndrome. Circulation 131, 2051–2060 (2015).
Chiang, C. et al. SpeedSeq: ultra-fast personal genome analysis and interpretation. Nat. Methods 12, 966–968 (2015).
Li, Y. et al. Resequencing of 200 human exomes identifies an excess of low-frequency non-synonymous coding variants. Nat. Genet. 42, 969–972 (2010).
The 1000 Genomes Project Consortium A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
The 1000 Genomes Project Consortium An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012).
Kocher, J. P. et al. The Biological Reference Repository (BioR): a rapid and flexible system for genomics annotation. Bioinformatics 30, 1920–1922 (2014).
Acknowledgements
K.K. is supported by the NIH (R00HL093212), NIH (R01AG043531), TriStem-Star Foundation (2013-049), Louis V. Gerstner, Jr. Young Investigators awards, Geoffrey Beene Junior Chair Award, Sidney Kimmel Scholar Award, Alfred W. Bressler Scholars Endowment Fund and MSKCC Society Fund. MSKCC Core Facilities are supported by NIH Cancer Center support grant P30 CA008748. R.Z. is supported by the UAB Development Fund. H.L. is supported by the NIH (CA196631-01A1) and Mayo Clinic Center for Individualized Medicine. C.-W.L. is supported by the NIH (R21HD081682). J.J.C. is supported by the HHMI and NIH (R24DK092760). S.L. is supported by the HHMI and NIH (P01CA087497). J.K. is supported by the NIH (R01GM112722). H.F. is supported by the TriStem-Star Foundation (2013-049). J.A. acknowledges support from the medical faculty of Heinrich Heine University, Düsseldorf. T.B. is supported by the MSKCC Single Cell Sequencing Initiative, William and Joyce O’Neil Research Fund and STARR visiting fellows programme. S.G. is supported by the American Italian Cancer Foundation and a Women in Science Rockefeller fellowship.
Author information
Authors and Affiliations
Contributions
M.S., R.Z., and K.K. conceived the experimental plan. M.S., P.Y., T.B., L.D., J.W., S.G., Z.L., M.B., R.Z. and K.K. carried out the experiments. C.C., T.B., S.B., C.Z., C.A.R., J.K. and H.L. carried out computational analysis. M.S., C.C., P.Y., T.B., C.A.R., S.B., J.A., H.F., J.K., S.L., J.J.C., C.-W.L., H.L., R.Z. and K.K. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Pluripotency of Y-iPSC, A-iPSC-ZSCAN10, and A-iPSC.
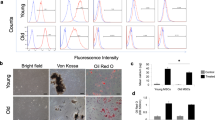
(a) Teratoma formation assay for ESC, Y-iPSC, A-iPSC-ZSCAN10, and A-iPSC. H/E staining was carried out with harvested teratoma to demonstrate the potential for tissue differentiation to three germ layers. Scale bar = 400 μm. (b) AP staining. (c) OCT4 expression levels in embryonic fibroblasts (MEF), ESC, Y-iPSC, A-iPSC-ZSCAN10, and A-iPSC by qPCR. Three representative independent clones of each cell line are shown. (d) Immunostaining for SSEA-1 and NANOG in ESC, Y-iPSC, A-iPSC-ZSCAN10, and A-iPSC. Nuclei were stained with DAPI. No NANOG expression was detected in fibroblasts, whereas iPSC from B6CBA expressed NANOG at low levels1. (e) Generation of chimeric mice by injection of Y-iPSC, A-iPSC, and A-iPSC-ZSCAN10 with GFP+ marker in E12.5 embryos. Two clones of each cell type were used to test the chimerism. Placentas/placental fetal membranes (red arrows) were included in the images as negative controls. Images on the TRITC filter were included to verify minimal auto-fluorescence signals. (f) Confirmation of transgene silencing in Y-iPSC, A-iPSC-ZSCAN10, and A-iPSC. Silencing of transgene expression (OCT4, SOX2, KLF4, and MYC) was confirmed by qPCR and RT-PCR using 1 μg of RNA and the primers specified in Methods. Shown here are three representative independent clones of each cell line. Uninfected fibroblasts were used as a negative control and day 3 fibroblasts transfected with Yamanaka factors were used as a positive control. No detectable expression of the four transgenes was observed after 40 cycles of amplification. We detected the signal in positive controls of OCT4 in PCR cycle 22.4, SOX2 in PCR cycle 18.5, KLF4 in PCR cycle 16, MYC in PCR cycle 17, and ACTIN in PCR cycle 13.5. (g) An example of normal karyotype in cytogenetic analysis of ESC, ntESC, Y-iPSC, A-iPSC-ZSCAN10, and A-iPSC. All three clones of ntESC showed a normal karyotype. (h) An example of a detailed analysis of chromosome instability in A-iPSC. (i, j) DNA damage response of A-iPSC (n = 10 independent clones in each cell type) generated from lung and bone marrow with pATM after phleomycin treatment (2 h, 30 μg ml−1). qPCR of ZSCAN10 mRNA levels (showing poor activation of ZSCAN10 expression) and GSS mRNA levels (indicating excessive expression) in A-iPSC generated from lung (n = 12) and bone marrow (n = 9). Error bars indicate standard error of the mean of the number of clones indicated above. Statistical significance was determined by two-sided t-test. Detailed information provided in Supplementary Table 5.
Supplementary Figure 2 Analysis pipeline to identify ZSCAN10 and GSS.
(a) ZSCAN10. Initially, 59 core pluripotency genes derived from a pluripotency network analysis were retrieved2 and filtered based on differential expression in A-iPSC and somatic cells (low) versus Y-iPSC or ESC (high) (96 genes with more than 2-fold difference), which narrowed down the candidates to eight genes (ZSCAN10, GBX2, SAL4, TCFAP2C, MYBL2, EVX1, OTX2, and MSH6). We cross-referenced each candidate gene that associated with the DNA damage response and genomic stability, and identified the top candidate, ZSCAN10, which was previously reported to have a regulatory link with ATM, GSS, p53, PARP, PLK1, and ZSCAN43,4,5,6. We confirmed differential expression of ZSCAN10 by qPCR in both mouse and human cells, and functionally assessed the recovery of the DNA damage response/genomic stability in this report. (b) Ontology analysis of genes with greater than two fold differential expression in A-iPSC versus Y-iPSC by Panther Go-Slin Ontology analysis tool (http://www.pantherdb.org). (c) Heatmap showing hierarchical clustering of differentially expressed genes between A-iPSC-ZSCAN10 and A-iPSC in the DNA damage response pathway. The gene list was extracted from Gene Ontology-GO (GO:0006974, http://www.geneontology.org/). Bar indicates the z-score scaled gene expression levels. Top genes include TLK1, PRPF19, APEX1, UNG, RAD52, BCCIP, GTF2H1, PRPF19, POLI, MSH3, RAD54L, BRE, FANCG, XRCC3, RAD51, DCLRE1C, and FANCL. Note that expression of ZSCAN10 in A-iPSC is sufficient to make A-iPSC-ZSCAN10 cluster together with ESC and Y-iPSC for a group of genes within the DNA damage response pathway. (d) GSS. To identify the ZSCAN10 targets involved in the DNA damage response defect in A-iPSC, we cross-referenced (1) differentially expressed genes in Y-iPSC/ESC and A-iPSC (634 genes with more than 2-fold difference), (2) genes with altered expression in A-iPSC compared with A-iPSC-ZSCAN10 (narrowed down to 464 genes), (3) the gene list of the ZSCAN10 targeted binding regions from those previously reported in ChIP-on-chip analysis in ESC5 (narrowed down to 39 genes), and (4) the list of genes extracted from Gene Ontology-GO involved in the DNA damage response-apoptosis-antioxidant-ROS-genomic stability-cell death pathway (GO:0006974, GO:0072593, GO:0048039, GO:0043295, GO:0034699, GO:0032275, GO:0031405, GO:0008431, GO:0008379, GO:0006776, GO:0006744, GO:0006915, GO:0001523, GO:0008219, GO:0012501, GO:0000302, GO:1903409, GO:0043066, GO:0097752). This narrowed down the list to eight candidate genes (BCCIP, PLAC8, DHODH, ESCO2, RASSF1, GSS, GDF15, MKNK2). Because cellular glutathione level is a well-established regulator of the DNA damage response and genomic stability30,31,32,33, we hypothesized that a glutathione imbalance contributes to the observed defects in A-iPSC. Among the differentially expressed glutathione regulatory genes, we identified glutathione synthetase (GSS), which is one of the eight genes directly bound by ZSCAN10, as the top candidate. The differential expression of GSS was confirmed by qPCR in both mouse and human cells, and the capacity to recovery of DNA damage response/genomic stability was functionally assessed in this report. (e) Heatmap shows hierarchical clustering analysis of differentially expressed genes between A-iPSC-ZSCAN10 and A-iPSC in the glutathione synthesis pathway. The gene list is extracted from Gene Ontology-GO (GO:0006749, http://www.geneontology.org). Color bar indicates the z-score scaled gene expression levels. Top genes include GSTP1, GSTP2, GSTT1, GSTT2, GSTT3, GSTA1, GSTK1, MGST1, GLRX2, and GSS. Note that expression of ZSCAN10 in A-iPSC is sufficient to make A-iPSC-ZSCAN10 cluster together with ESC and Y-iPSC for a group of the genes within the glutathione synthesis pathway, further supporting GSS as a top candidate.
Supplementary Figure 3 Extended characterization of the DNA damage response of A-iPSC and recovery by ZSCAN10 expression.
(a) ZSCAN10 expression during iPSC reprogramming. Using serial live imaging of fibroblasts derived from ESC undergoing reprogramming7 in which the tdTomato reporter gene was inserted in 3′ end of ZSCAN10 gene by CRISPR targeting, we observed that ZSCAN10 expression started on day 6 (8 of 11 clones documented), but 3 of 11 clones did not. Starting on day 8, all clones showed tdTomato-ZSCAN10 reporter signals, which increased on day 11 when the first colonies were formed. Images on the GFP filter were included to verify minimal auto-fluorescence signals. (b) Immunostaining for ZSCAN10 in ESC, Y-iPSC, A-iPSC-ZSCAN10, and A-iPSC. Nuclei were stained with DAPI. (c) DNA damage response of ntESC (three independent clones) with γH2AX and p53 after phleomycin treatment (2 h, 30 μg ml−1). (d) Enlarged images of the immunohistochemistry showing low γ-H2AX (phosphorylated H2AX) in A-iPSC after phleomycin treatment (2 h, 30 μg ml−1) and recovery of γ-H2AX signal with ZSCAN10 expression. (e) No difference in γ-H2AX staining in A-iPSC at a standard (30 min) and extended (6 h) recovery time after phleomycin treatment (2 h, 30 μg ml−1). Scale bar = 10 μm. (f) Ratio of γ-H2AX-positive cells to DAPI-stained nuclei quantified by immunostaining. Only cells showing punctate γ-H2AX foci were counted. The number of colonies counted is indicated in each group. Error bars indicate standard error of the mean of ten biological replicates in each sample group (n = 10). Detailed information provided in Supplementary Table 5. (g) Growth rate of ESC, Y-iPSC, A-iPSC-ZSCAN10, and A-iPSC. 10,000 cells per line were plated in triplicate in 24-well dishes, and the numbers of cells were counted every 24 h for 3 days (n = 6). Error bars indicate standard error of the mean of three biological replicates with two independent clones in each sample group. (h) Telomere length analysis of iPSC. Telomere length assay was carried out by quantitative PCR as previously described8. β-actin was used as a control for single copy gene per sample. Error bars indicate standard error of the mean of four independent clones in A-iPSC-ZSCAN10 (n = 4) and A-iPSC (n = 4), and three independent clones in Y-iPSC (n = 3). Statistical significance was determined by two-sided t-test. Detailed information provided in Supplementary Table 5. (i) Expression level of ZSCAN10 after shRNA expression, determined by qPCR. The red dashed line denotes data that supports the findings presented in the manuscript (Fig. 2e). Error bars indicate standard error of the mean of two technical replicates with three independent clones in each sample group (n = 3). Statistical significance was determined by two-sided t-test. (j) Impaired ZSCAN10 expression and DNA damage response of Y-iPSC and A-iPSC-ZSCAN10 clones with poor apoptotic response. Poor expression of ZSCAN10 in the outlier clones of Y-iPSC and A-iPSC-ZSCAN10 with poor apoptotic response (red circles in Fig. 1c) were determined by qPCR. Error bars indicate standard error of the mean of two technical replicates with three biological replicates in each sample group (n = 3). (k) Impaired ATM-mediated DNA damage response of the outlier clones of Y-iPSC and A-iPSC-ZSCAN10 are shown by immunoblot after phleomycin treatment (2 h, 30 μg ml−1). (l) Poor ATM-mediated DNA damage response of A-iPSC after H2O2 treatment, and recovery with ZSCAN10 expression. ESC and Y-iPSC, but not A-iPSC, show an increase in pATM level after H2O2 treatment (tert-butyl hydrogen peroxide; stable chemical form of H2O2, 350 μM, 30 min in PBS). The ATM response to H2O2 treatment in A-iPSC is recovered by transient expression of ZSCAN10. This result correlates with strong ROS scavenging activity in A-iPSC, and recovery by transient expression of ZSCAN10. (m) DNA damage response of Y-iPSC generated from neonatal skin fibroblasts. ESC with ATM −/− H2AX −/− mutations, iPSC with p53 −/− mutation, and A-iPSC were used as controls. Both Y-iPSC derived from embryonic skin fibroblasts and Y-iPSC derived from neonatal skin fibroblasts showed normal DNA damage response after phleomycin treatment (2 h, 30 μg ml−1).
Supplementary Figure 4 Recovery of ROS-glutathione homeostasis in A-iPSC by reduction (shRNA-GSS) of excessively activated GSS and disruption of ROS-glutathione homeostasis in Y-iPSC by overexpression of GSS.
(a) Expression levels of GSS after shRNA knockdown of ZSCAN10 in Y-iPSC. The red dashed line denotes data that supports the findings presented in the manuscript (Fig. 2e). Error bars indicate standard error of the mean of two technical replicates with three independent clones in each sample group (n = 3). Statistical significance was determined by two-sided t-test. (b) Confirmation of the ZSCAN10 binding activity to the GSS promoter that reported by Yu et al., J. Biol. Chem., 2009 using ChIP–qPCR14. Oct4 promoter region was used as a positive control and an 80-bp genomic region on chromosome 4 was used as a negative control. Error bars indicate standard error of the mean of five replicates for AiPSC and YiPSC (n = 5) and three replicates for positive/negative controls (n = 3). Statistical significance was determined by two-sided t-test. Detailed information provided in Supplementary Table 5. (c) Expression levels of GSS after shRNA knockdown of GSS in A-iPSC. Error bars indicate standard error of the mean of two technical replicates with three independent clones in each sample group (n = 3). Statistical significance was determined by two-sided t-test. Detailed information provided in Supplementary Table 5. (d) Oxidation capacity of glutathione in A-iPSC, A-iPSC-shRNA-GSS, Y-iPSC, and Y-iPSC-GSS. Quantification of reduced glutathione (GSH) and oxidized glutathione (GSSG) was measured to determine the total glutathione level (maximum oxidation capacity). Glutathione analysis was conducted with the Glutathione Fluorometric Assay (Biovision, K264-100). Error bars indicate standard error of the mean of two technical replicates with four independent clones in A-iPSC-shRNA GSS (n = 4) and two technical replicates with seven independent clones in A-iPSC-shRNA GSS (n = 7). Statistical significance was determined by two-sided t-test. (e) H2O2 scavenging activity in ESC, Y-iPSC, Y-iPSC-GSS, A-iPSC, A-iPSC-ZSCAN10, and A-iPSC-shRNA-GSS. A cellular reactive oxygen species assay kit (Abcam, ab113851) was used to measure the H2O2 scavenging activity. Excessive H2O2 scavenging activity in A-iPSC was recovered by shRNA-GSS expression. The excessive H2O2 scavenging activity seen in A-iPSC was recapitulated by GSS overexpression in Y-iPSC. Mean ± standard deviation is plotted for four replicates from each condition. Error bars indicate standard error of the mean of three biological replicates with three independent clones in Y-iPSC-GSS and A-iPSC-shRNA GSS (n = 9). Statistical significance was determined by two-sided t-test. The red dashed line denotes data that supports the findings presented in the manuscript (Fig. 3f and 3 h). (f) Expression levels of GSS in Y-iPSC after overexpression of GSS. Error bars indicate standard error of the mean of two technical replicates with three independent clones in each sample group (n = 3). Statistical significance was determined by two-sided t-test.
Supplementary Figure 5 Pluripotency of human Y-hiPSC, A-hiPSC-ZSCAN10, and A-hiPSC.
(a) Teratoma formation assay for hESC (H9), Y-hiPSC, A-hiPSC-ZSCAN10, and A-hiPSC. Hematoxylin and eosin staining of teratomas derived from immunodeficient mice injected with hESC (H9), Y-hiPSC, A-hiPSC, or A-hiPSC-ZSCAN10 reveals differentiation to all three embryonic germ layers, including cartilage (mesoderm), muscle (mesoderm), neuroepithelial rosettes (ectoderm), brain (ectoderm), intestinal epithelium (endoderm), and respiratory epithelium (endoderm). Scale, 100 μm. (b) Human pluripotent gene expression in hESC (H9), Y-hiPSC, A-hiPSC, and A-hiPSC-ZSCAN10 were confirmed by flow cytometry analysis (TRA181, SSEA3, and SSEA4) and qPCR (OCT4). Error bars on qPCR indicate standard error of the mean of two replicates with three independent clones in each sample group (n = 6). Fibroblasts were used as a negative control. (c) Quantification by image-analysis of apoptotic response by DNA fragmentation assay after phleomycin treatment. Error bars indicate standard error of the mean of n = 10 biological replicates. Statistical significance was determined by two-sided t-test. (d,e) DNA damage response with ATM after phleomycin treatment (2 h, 30 μg ml−1) in A-hiPSC-AG4 clones that were reprogrammed with lentivirus expressing hOCT4, hSOX2, and hKLF4 without hMYC expression (d) and with integration-free episomal vectors (e). (f) Immunoblot showing impaired ATM DNA damage response in Y-hiPSC (H1OGN) with ZSCAN10 shRNA expression in three independent clones after phleomycin treatment (2 h, 30 μg ml−1). (g) Confirmation of the ZSCAN10 binding activity to the consensus binding motif on the GSS promoter that reported by Yu et al. J. Biol. Chem., 2009 using ChIP-qPCR14. Error bars indicate standard error of the mean of three replicates for AiPSC and YiPSC (n = 3) and three replicates for positive/negative controls (n = 3) Statistical significance was determined by two-sided t-test followed by post-hoc Holm–Bonferroni correction for a significance level of 0.05, ∗ indicates significant and NS: not significant. Oct4 promoter region was used as a positive control and an 94 bp region on chromosome 14 was used as a negative control. Detailed information provided in Supplementary Table 5. (h) Copy number profiling analysis of various hiPSC, including examples of non-rearranged A-hiPSC, rearranged A-hiPSC, non-rearranged A-hiPSC-ZSCAN10, and non-rearranged Y-hiPSC. (i) Representative high-resolution G-banded karyotyping analysis of human iPSC clones. Y-hiPSC, A-hiPSC, and A-hiPSC ZSCAN10 showed normal karyotypes. However, A-hiPSC showed a higher frequency of cytogenetic abnormalities (Fig. 5e). A-hiPSC-JA also had an abnormal karyotype, with partial deletion of the p-arm of chromosome 20 and a lost Y chromosome. (j) Genes mutated in the coding region of A-hiPSC and A-hiPSC-ZCAN10. Number of variants found with a sequencing depth of at least 20 and a consensus quality score of at least 20.
Supplementary Figure 6 Higher somatic cell ROS among the tissue donors as a causative origin of the genomic instability in A-iPSC and recovery by glutathione treatment in the early stage of A-iPSC reprogramming.
(a,b) Somatic cell ROS measured by MitoSOX staining. Mitochondrial Superoxide Indicator, MitoSOX Red dye (ThermoFisher, M36008) was used to measure somatic cell ROS from young somatic cells (Y-SC) from B6CBA mouse and aged somatic cells (A-SC) from B6129 and B6CBA mice (a), and human young somatic cells (Y-SC) from MRC5 donor and human aged somatic cells (A-SC) from LS and AG4 donors (b). Reduced level of the somatic cell ROS with the treatment of the stabilized form of glutathione chemical (3 mM of glutathione reduced ethyl ester, CAT #G-275-500, GoldBio) for three days in the media in A-SC from AG4 donor (A-SC-AG4-glutathione) (b). Scale bar indicates 100 μm. (c,d) Quantification of the MitoSOX staining (ROS) level using image-based quantification (ImageJ software) from the samples in Supplementary Fig. 6A, B. Error bars indicate standard error of the mean of independent colonies (n = 10). Statistical significance was determined by unpaired two-sided t-test. (e–f) Representative phenotypes of the reprogrammed iPSC from the donor somatic cells from Supplementary Fig. 6A, B from mouse and human donors. A-SC-AG4 somatic cells with the reduced ROS by the treatment of the glutathione reduced ethyl ester in Supplementary Fig. 6B, D was studied. (g) Immunoblot of pATM showing that A-iPSC with glutathione treatment recover the DNA damage response in the biologically independent clones after phleomycin treatment. A-iPSC were generated with the treatment of 3 mM glutathione reduced ethyl ester prior to and during the early stage of reprogramming (from one day before reprogramming virus infection to 10 days post reprogramming virus infection). (h) Copy number profiling analysis of human A-iPSC with glutathione treatment from 10 clones (upper panel). Schematic diagrams represent 10 non-rearranged A-iPSC with glutathione treatment, compared with seven rearranged A-iPSC from 11 clones (lower panel) without glutathione treatment. A-hiPSC (n = 11 (7/11), p = 0.64) and A-hiPSC-glutathione (n = 10 (0/10), p∗ < 4E-5). The p values are the observed (p) and estimated likelihoods (p∗) of detecting no rearrangements in the absence of lineage effects using a binomial distribution, respectively. (I) qPCR of ZSCAN10. Error bars indicate standard error of the mean of two replicates with two independent clones in each sample group in Supplementary Fig. 6G. Statistical significance was determined by two-sided t-test (n = 4). (J) qPCR of GSS. Error bars indicate standard error of the mean of two replicates with two independent clones in each sample group in Supplementary Fig. 6G. Statistical significance was determined by two-sided t-test (n = 4).
Supplementary information
Supplementary Information
Supplementary Information (PDF 3072 kb)
Supplementary Table 1
Supplementary Information (XLSX 18 kb)
Supplementary Table 2
Supplementary Information (XLSX 9 kb)
Supplementary Table 3
Supplementary Information (XLSX 10 kb)
Supplementary Table 4
Supplementary Information (XLSX 11 kb)
Supplementary Table 5
Supplementary Information (XLSX 39 kb)
Rights and permissions
About this article
Cite this article
Skamagki, M., Correia, C., Yeung, P. et al. ZSCAN10 expression corrects the genomic instability of iPSCs from aged donors. Nat Cell Biol 19, 1037–1048 (2017). https://doi.org/10.1038/ncb3598
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3598
This article is cited by
-
A ride through the epigenetic landscape: aging reversal by reprogramming
GeroScience (2021)
-
Glis1 facilitates induction of pluripotency via an epigenome–metabolome–epigenome signalling cascade
Nature Metabolism (2020)
-
An Insight into Reprogramming Barriers to iPSC Generation
Stem Cell Reviews and Reports (2020)
-
Retention of Somatic Memory Associated with Cell Identity, Age and Metabolism in Induced Pluripotent Stem (iPS) Cells Reprogramming
Stem Cell Reviews and Reports (2020)
-
Maintaining genomic stability in pluripotent stem cells
Genome Instability & Disease (2020)