Abstract
The Ndc80 complex (Ndc80, Nuf2, Spc24 and Spc25) is a highly conserved kinetochore protein essential for end-on anchorage to spindle microtubule plus ends and for force generation coupled to plus-end polymerization and depolymerization. Spc24/Spc25 at one end of the Ndc80 complex binds the kinetochore. The N-terminal tail and CH domains of Ndc80 bind microtubules, and an internal domain binds microtubule-associated proteins (MAPs) such as the Dam1 complex. To determine how the microtubule- and MAP-binding domains of Ndc80 contribute to force production at the kinetochore in budding yeast, we have inserted a FRET tension sensor into the Ndc80 protein about halfway between its microtubule-binding and internal loop domains. The data support a mechanical model of force generation at metaphase where the position of the kinetochore relative to the microtubule plus end reflects the relative strengths of microtubule depolymerization, centromere stretch and microtubule-binding interactions with the Ndc80 and Dam1 complexes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wang, H. W. et al. Architecture and flexibility of the yeast Ndc80 kinetochore complex. J. Mol. Biol. 383, 894–903 (2008).
Ciferri, C. et al. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133, 427–439 (2008).
Suzuki, A., Badger, B. L. & Salmon, E. D. A quantitative description of Ndc80 complex linkage to human kinetochores. Nat. Commun. 6, 8161 (2015).
Wei, R. R., Al-Bassam, J. & Harrison, S. C. The Ndc80/HEC1 complex is a contact point for kinetochore–microtubule attachment. Nat. Struct. Mol. Biol. 14, 54–59 (2007).
Hsu, K. S. & Toda, T. Ndc80 internal loop interacts with Dis1/TOG to ensure proper kinetochore-spindle attachment in fission yeast. Curr. Biol. 21, 214–220 (2011).
Maure, J. F. et al. The Ndc80 loop region facilitates formation of kinetochore attachment to the dynamic microtubule plus end. Curr. Biol. 21, 207–213 (2011).
Zhang, G. et al. The Ndc80 internal loop is required for recruitment of the Ska complex to establish end-on microtubule attachment to kinetochores. J. Cell Sci. 125, 3243–3253 (2012).
Lampert, F., Mieck, C., Alushin, G. M., Nogales, E. & Westermann, S. Molecular requirements for the formation of a kinetochore-microtubule interface by Dam1 and Ndc80 complexes. J. Cell Biol. 200, 21–30 (2013).
Nogales, E. & Ramey, V. H. Structure-function insights into the yeast Dam1 kinetochore complex. J. Cell Sci. 122, 3831–3836 (2009).
Cheeseman, I. M., Enquist-Newman, M., Muller-Reichert, T., Drubin, D. G. & Barnes, G. Mitotic spindle integrity and kinetochore function linked by the Duo1p/Dam1p complex. J. Cell Biol. 152, 197–212 (2001).
Enquist-Newman, M. et al. Dad1p, third component of the Duo1p/Dam1p complex involved in kinetochore function and mitotic spindle integrity. Mol. Biol. Cell 12, 2601–2613 (2001).
Janke, C., Ortiz, J., Tanaka, T. U., Lechner, J. & Schiebel, E. Four new subunits of the Dam1-Duo1 complex reveal novel functions in sister kinetochore biorientation. EMBO J. 21, 181–193 (2002).
Hofmann, C. et al. Saccharomyces cerevisiae Duo1p and Dam1p, novel proteins involved in mitotic spindle function. J. Cell Biol. 143, 1029–1040 (1998).
Li, Y. et al. The mitotic spindle is required for loading of the DASH complex onto the kinetochore. Genes Dev. 16, 183–197 (2002).
Tien, J. F. et al. Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by aurora B. J. Cell Biol. 189, 713–723 (2010).
Sarangapani, K. K., Akiyoshi, B., Duggan, N. M., Biggins, S. & Asbury, C. L. Phosphoregulation promotes release of kinetochores from dynamic microtubules via multiple mechanisms. Proc. Natl Acad. Sci. USA 110, 7282–7287 (2013).
Franck, A. D. et al. Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis. Nat. Cell Biol. 9, 832–837 (2007).
Inoue, S. & Salmon, E. D. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol. Biol. Cell 6, 1619–1640 (1995).
Gardner, M. K. et al. Tension-dependent regulation of microtubule dynamics at kinetochores can explain metaphase congression in yeast. Mol. Biol. Cell 16, 3764–3775 (2005).
McIntosh, J. R. et al. Conserved and divergent features of kinetochores and spindle microtubule ends from five species. J. Cell Biol. 200, 459–474 (2013).
Volkov, V. A. et al. Long tethers provide high-force coupling of the Dam1 ring to shortening microtubules. Proc. Natl Acad. Sci. USA 110, 7708–7713 (2013).
Slep, K. C. & Vale, R. D. Structural basis of microtubule plus end tracking by XMAP215, CLIP-170, and EB1. Mol. Cell 27, 976–991 (2007).
Tanaka, K. et al. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature 434, 987–994 (2005).
Joglekar, A. P., Bloom, K. & Salmon, E. D. In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Curr. Biol. 19, 694–699 (2009).
Haase, J. et al. A 3D map of the yeast kinetochore reveals the presence of core and accessory centromere-specific histone. Curr. Biol. 23, 1939–1944 (2013).
Ohashi, T., Galiacy, S. D., Briscoe, G. & Erickson, H. P. An experimental study of GFP-based FRET, with application to intrinsically unstructured proteins. Protein Sci. 16, 1429–1438 (2007).
Lawrimore, J., Bloom, K. S. & Salmon, E. D. Point centromeres contain more than a single centromere-specific Cse4 (CENP-A) nucleosome. J. Cell Biol. 195, 573–582 (2011).
Winey, M. & Bloom, K. Mitotic spindle form and function. Genetics 190, 1197–1224 (2012).
Verdaasdonk, J. S. et al. Centromere tethering confines chromosome domains. Mol. Cell 52, 819–831 (2013).
DeLuca, K. F., Lens, S. M. & DeLuca, J. G. Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J. Cell Sci. 124, 622–634 (2011).
Akiyoshi, B., Nelson, C. R., Ranish, J. A. & Biggins, S. Analysis of Ipl1-mediated phosphorylation of the Ndc80 kinetochore protein in Saccharomyces cerevisiae. Genetics 183, 1591–1595 (2009).
Suzuki, A., Badger, B. L., Wan, X., DeLuca, J. G. & Salmon, E. D. The architecture of CCAN proteins creates a structural integrity to resist spindle forces and achieve proper intrakinetochore stretch. Dev. Cell 30, 717–730 (2014).
Demirel, P. B., Keyes, B. E., Chaterjee, M., Remington, C. E. & Burke, D. J. A redundant function for the N-terminal tail of Ndc80 in kinetochore-microtubule interaction in Saccharomyces cerevisiae. Genetics 192, 753–756 (2012).
Pearson, C. G., Maddox, P. S., Zarzar, T. R., Salmon, E. D. & Bloom, K. Yeast kinetochores do not stabilize Stu2p-dependent spindle microtubule dynamics. Mol. Biol. Cell 14, 4181–4195 (2003).
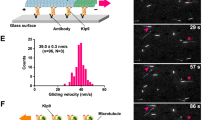
Pearson, C. G. et al. Measuring nanometer scale gradients in spindle microtubule dynamics using model convolution microscopy. Mol. Biol. Cell 17, 4069–4079 (2006).
Maddox, P. S., Stemple, J. K., Satterwhite, L., Salmon, E. D. & Bloom, K. The minus end-directed motor Kar3 is required for coupling dynamic microtubule plus ends to the cortical shmoo tip in budding yeast. Curr. Biol. 13, 1423–1428 (2003).
Marco, E. et al. S. cerevisiae chromosomes biorient via gradual resolution of syntely between S phase and anaphase. Cell 154, 1127–1139 (2013).
Grishchuk, E. L. et al. The Dam1 ring binds microtubules strongly enough to be a processive as well as energy-efficient coupler for chromosome motion. Proc. Natl Acad. Sci. USA 105, 15423–15428 (2008).
Westermann, S. et al. Formation of a dynamic kinetochore-microtubule interface through assembly of the Dam1 ring complex. Mol. Cell 17, 277–290 (2005).
Nicklas, R. B., Lee, G. M., Rieder, C. L. & Rupp, G. Mechanically cut mitotic spindles: clean cuts and stable microtubules. J. Cell Sci. 94, 415–423 (1989).
Chacon, J. M., Mukherjee, S., Schuster, B. M., Clarke, D. J. & Gardner, M. K. Pericentromere tension is self-regulated by spindle structure in metaphase. J. Cell Biol. 205, 313–324 (2014).
Shimogawa, M. M. et al. Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase. Curr. Biol. 16, 1489–1501 (2006).
Zaytsev, A. V. et al. Multisite phosphorylation of the NDC80 complex gradually tunes its microtubule-binding affinity. Mol. Biol. Cell 26, 1829–1844 (2015).
Powers, A. F. et al. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell 136, 865–875 (2009).
Lampert, F., Hornung, P. & Westermann, S. The Dam1 complex confers microtubule plus end-tracking activity to the Ndc80 kinetochore complex. J. Cell Biol. 189, 641–649 (2010).
Joglekar, A. P., Bloom, K. S. & Salmon, E. D. Mechanisms of force generation by end-on kinetochore-microtubule attachments. Curr. Opin. Cell Biol. 22, 57–67 (2010).
Dumont, S., Salmon, E. D. & Mitchison, T. J. Deformations within moving kinetochores reveal different sites of active and passive force generation. Science 337, 355–358 (2012).
Zaytsev, A. V., Sundin, L. J., DeLuca, K. F., Grishchuk, E. L. & DeLuca, J. G. Accurate phosphoregulation of kinetochore-microtubule affinity requires unconstrained molecular interactions. J. Cell Biol. 206, 45–59 (2014).
Acknowledgements
We would like to thank E. Yeh (UNC, USA), K. Plevock (UNC, USA), D. Pellman (Harvard, USA) and S. Biggins (Fred Hutch, USA) for critical reagents and valuable suggestions. We would also like to thank A. McAnish for providing the drawing tool for the model. This work was supported by the Uehara Memorial Foundation, Kazato Research Foundation, and Japan Society and Promotion of Science (A.S.), the Summer Undergraduate Research Fellowship from UNC (B.L.B.), and by R37GM024364 and R01GM24364 (E.D.S.), R37GM32238 (K.B.) and R01GM66014 (H.P.E.) from the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
A.S. performed entire experiments and analysed the data. A.S. and B.L.B. performed experiments for Figs 2–4 and Supplementary Figs 4 and 5. J.H. performed the experiments for Supplementary Figs 1d, 2e and 6e. T.O. and H.P.E. provided FRET probes and discussion of their use and interpretation. E.D.S. wrote the computer simulation of the mechanistic model. A.S., E.D.S. and K.B. designed all experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
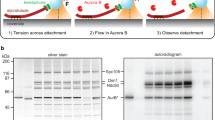
Supplementary Figure 4 The Ndc80 FRET sensor is located between the CH domain and the Loop domain.
(a) Method of establishment of the Ndc80FRET sensor cells. (b) PCR using primers in (a) amplified in control or Ndc80 FRET biosensor genome. (c) Representative images of mECFP and mYPet in Ndc80 FRET sensor cell, Ndc80-mECFP cell, and Ndc80-mYPet cell. (d) Confirmation of FRET probe position within the Ndc80 protein by the nm-scale heat-map method (see Methods). For this analysis, we established the Ndc80-GFP (M, at the aa 410) cell line, where GFP was inserted into the same position as the FRET probe. In short, the peak intensity (brightest pixel) of each fluorescent spot was determined and the coordinate of each spot was plotted relative to the spindle pole body (see Methods). The coordinates of over 100 images were used to generate a positional density map representing the distribution of Ndc80 as a fraction of the distance (nm) from the center of the spindle pole. (e) Representative images of GFP in Ndc80-GFP (C-terminal) cell, and Ndc80-GFP (M) cell (left). The ratio of GFP intensities in Ndc80-GFP cell and Ndc80-GFP (M) cell (right). n = 100 kinetochores. Error bars are SD from the means. Scale bars are 5 μm. The mean values were calculated using the data pooled from 2 independent experiments.
Supplementary Figure 5 The FRET Emission Ratio is proportional to FRET efficiency.
(a) Determination of FRET efficiency by imaging mixtures of cells, some containing the Ndc80 FRET sensor (yellow arrow) and some with mECFP only at the FRET sensor position in Ndc80 (red arrow) (see Methods). Enhanced contrast images show cell shape (right). (b) Measured values of FRET efficiency for control metaphase and late anaphase, and for cells treated with low-dose benomyl (55 μM) (see Supplementary Table 1 for details). n = 100 kinetochores. Error bars are SD from the means. The mean values were calculated using the data pooled from 3 independent experiments. (c) FRET efficiency versus Emission Ratio shows a linear dependency. (d) Representative FRET images of Ndc80 FRET sensor, Ndc80-mYPet, and Ndc80-mECFP in late anaphase cells (left). The cross excitation from mYPet and bleed through from mECFP was determined from the cell lines expressing these constructs, excited at CFP wavelength and emission measured at Ypet (FRET filter) or CFP (CFP filter) using Ndc80-mYPet (n = 400 kinetochores) and Ndc80-mECFP (n = 54 kinetochores) cells (right, see Methods for details). These values were used for the no-FRET bars in Fig. 2d. The mean values were calculated using the data pooled from 2 independent experiments. (e) The plots of mean distance from Cse4 (internal GFP fusion at aa position 81) to Ndc80 (N-terminal GFP fusion) from prometaphase through late anaphase. Anaphase onset is the interval between 700–799 and 800–899 bin. The distance between Cse4 and Ndc80 was determined from the statistical distribution (heat maps) of brightest pixels from hundreds of images as described in the Methods and Figure Legend (Supplementary Fig. 1d). (b) The mean distance from Cse4 and Ndc80 in metaphase and late anaphase. The mean values were calculated using the data pooled from 2 independent experiments. Scale bars are 1 μm (a), 5 μm (d). Error bars are SD from the means.
Supplementary Figure 6 The description of N-terminal tail deletion mutants.
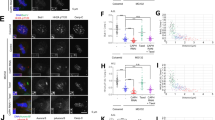
(a) Diagram showing construction of the partial (70 Del) and the whole N-terminal tail deletion (112 Del) mutants. To maintain Ndc80 FRET biosensor protein level, we expressed Ndc80 mutants from the endogenous Ndc80 promoter (left, also see Supplementary Table 3). (b) The PCR using primers in (left) amplified in control, 70 Del, or 112 Del (right). (c) The population of interphase, prometaphase, metaphase, anaphase, telophase, and apoptotic cells in control, 70 Del, and 112 Del mutants. n = 1037 (control), 1103 (70 Del), 1074 (112 Del). The mean values were calculated using the data pooled from 2 independent experiments.
Supplementary Figure 7 Integrated intensity measurements of sister kinetochore clusters during metaphase.
(a) The ratio of K1/K2 for Bim1-GFP, another microtubule plus-end binding protein, also fluctuated during metaphase and fluctuations dropped when cells were treated with low dose benomyl. (b) The FRET, mECFP, and mYPet images used for analysis in Fig. 2b. (c) The ratio of FRET Emission (K1/K2) changed similar to the ratio of FRET Emission Ratios (K1/K2). (d) Additional 4 examples showing that the level of Stu2-tdTomato fluctuates in opposite direction compared to the ratio of FRET Emission (K1/K2) during metaphase. 5 complete datasets, including Fig. 4c, were shown from 12 analyses. (e) Two examples of the ratio of K1/K2 for Ndc80-GFP integrated intensities obtained from time-lapse images of control metaphase. Scale bars are 1 μm (a,e) and 5 μm (b). We analyzed 12 cells from 3 independent experiments.
Supplementary Figure 8 FRAP analysis using Ndc80-GFP stain in prometaphase, metaphase, and late anaphase cells.
Representative time-lapse images of Ndc80-GFP cells before and after photo-bleaching in prometaphase, metaphase, and late anaphase (top). †was the target sister kinetochore cluster. Sister kinetochore cluster (#) in late anaphase was out of focus. The plots of integrated GFP intensities (bottom) in prometaphase, metaphase, and late anaphase were normalized GFP intensity before photo-bleaching. Scale bar is 1.5 μm. We analyzed 12 cells from 3 independent experiments.
Supplementary Figure 9 The mechanical model simulation results as presented in 5, but at higher time resolution.
(a) Computer simulations of mechanical model in Fig. 5 for 100 instead of 600 s along the x-axis for wild type (top), low dose benomyl to reduce dynamicity (2nd panels), N-terminal tail deletion (3rd panels) and cells with higher Dam1 Drag force (dam-765) (bottom) (see Supplementary Table 2 for detail parameters). (b) Representative captured images at single time point in the middle of simulation from Movies 1–4 including control, low dose benomyl, whole N-terminal tail deletion (112 Del), and higher Dam1 drag force (proposed for dam1-765 mutant). (c) Representative images of Cse4-GFP (an inner kinetochore marker) and Nuf2-GFP in metaphase of control or dam1-765 cells (left). The equal ratio of GFP intensities for each protein between sister kinetochore clusters in control and dam1-765 cells (right). The GFP intensity at kinetochore cluster (K1) was normalized by the value of the opposite sister kinetochore cluster (K2) (K2 > K1), then the value (K1/K2) in dam1-765 was normalized by the value in control (K1/K2). If the sister kinetochores are properly separated, the value should be close to 1. n = 100 kinetochore clusters. The mean values were calculated using the data pooled from 3 independent experiments. (d) Representative images of Spc29-RFP (a pole marker) and Cse4-GFP (a kinetochore marker) in metaphase of control or dam1-765 cells (left). Histograms of individual measurements of the separations between Cse4-Cse4 and Spc29-Spc29 normalized by the mean separation between Spc29-Spc29 (right). Sister kinetochores become further separated and closer on average to their poles in dam1-765 cells compared to controls. n = 100 kinetochore pairs. The mean values were calculated using the data pooled from 3 independent experiments. (e) Histograms of the separations between LacO/Lac1-GFP in sister chromosomes inserted 6.8 kbp from Cen15 in control or Dam1-765 cells. The dam1-765 mutant cells exhibit hyper-centromere chromatin stretch. n = 100 Lac-O/I pairs. The mean values were calculated using the data pooled from 3 independent experiments. Scale bars are 5 μm (c) and 1 μm (d). Error bars are SD from the means.
Supplementary Figure 10 Summary schematic of Force Coupler model in budding yeast metaphase.
(a) The kinetic diagram for the dynamics of kinetochores relative to the plus ends of their kMTs in controls and cells with higher Dam1 drag force proposed for dam1-765 in the mechanical model proposed in Fig. 5 and simulated in Figs 5c and 6a. In the control cells, a kinetochore almost always tracks its kMT plus-end during polymerization and depolymerization (left). The mean position of a kinetochore from its pole is almost equal to the mean length of its kMT. In contrast, for cells with higher Dam1 drag force, during depolymerization peeling protofilaments at a depolymerizing kMT-end pushes the kinetochore toward the minus-end (Step 1). Upon switch to polymerization, force from centromere stretch is insufficient to pull the kinetochore toward the MT plus-end at the rate of polymerization because of the higher Dam1 drag force (dam1-765) (Step 2). As a consequence of repeated cycles of MT dynamic instability, the average position of the kinetochore becomes closer to the MT minus-end at the spindle pole compared to the mean length of its kMT (Step 2). (b) During depolymerization, forces from peeling protofilaments push the Dam1 and Ndc80 complexes along kMTs toward the pole to stretch the centromere (see Fig. 5a). During polymerization, forces from centromere stretch pull the Dam1 and Ndc80 complexes along kMTs away from the pole (Fig. 5a).
Supplementary information
Supplementary Information
Supplementary Information (PDF 3861 kb)
Kinetics, control.
Movie derived from 600 s duration simulations presented in Fig. 5c, showing kinetics for all 16 sister kinetochore pairs and their centromere stretch. (MP4 1728 kb)
Kinetics, low dose Benomyl.
Movie derived from 600 s duration simulations presented in Fig. 5c, showing kinetics for all 16 sister kinetochore pairs and their centromere stretch. (MP4 620 kb)
Kinetics, whole N-terminal tail deletion (112 Del).
Movie derived from 600 s duration simulations presented in Fig. 5c, showing kinetics for all 16 sister kinetochore pairs and their centromere stretch. (MP4 1669 kb)
Kinetics, dam1-765 mutant (proposed 10-fold higher Dam1 drag force).
Movie derived from 600 s duration simulations presented in Fig. 6a, showing kinetics for all 16 sister kinetochore pairs and their centromere stretch. (MP4 1278 kb)
Rights and permissions
About this article
Cite this article
Suzuki, A., Badger, B., Haase, J. et al. How the kinetochore couples microtubule force and centromere stretch to move chromosomes. Nat Cell Biol 18, 382–392 (2016). https://doi.org/10.1038/ncb3323
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3323
This article is cited by
-
Ubiquitin-conjugating enzyme E2C (UBE2C) is a prognostic indicator for cholangiocarcinoma
European Journal of Medical Research (2023)
-
Probing stress-regulated ordering of the plant cortical microtubule array via a computational approach
BMC Plant Biology (2023)
-
Bridgin connects the outer kinetochore to centromeric chromatin
Nature Communications (2021)
-
Towards complete and error-free genome assemblies of all vertebrate species
Nature (2021)
-
Development of a risk scoring system for evaluating the prognosis of patients with Her2-positive breast cancer
Cancer Cell International (2020)