Abstract
Successful generation of induced pluripotent stem cells entails a major metabolic switch from mitochondrial oxidative phosphorylation to glycolysis during the reprogramming process. The mechanism of this metabolic reprogramming, however, remains elusive. Here, our results suggest that an Atg5-independent autophagic process mediates mitochondrial clearance, a characteristic event involved in the metabolic switch. We found that blocking such autophagy, but not canonical autophagy, inhibits mitochondrial clearance, in turn, preventing iPSC induction. Furthermore, AMPK seems to be upstream of this autophagic pathway and can be targeted by small molecules to modulate mitochondrial clearance during metabolic reprogramming. Our work not only reveals that the Atg5-independent autophagy is crucial for establishing pluripotency, but it also suggests that iPSC generation and tumorigenesis share a similar metabolic switch.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Zhou, H. et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4, 381–384 (2009).
Boya, P., Reggiori, F. & Codogno, P. Emerging regulation and functions of autophagy. Nat. Cell Biol. 15, 713–720 (2013).
Mizushima, N. et al. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J. Cell Biol. 152, 657–668 (2001).
Kuma, A. et al. The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 (2004).
Wang, S. et al. Transient activation of autophagy via Sox2-mediated suppression of mTOR is an important early step in reprogramming to pluripotency. Cell Stem Cell 13, 617–625 (2013).
Wernig, M. et al. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat. Biotechnol. 26, 916–924 (2008).
Klionsky, D. J. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8, 931–937 (2007).
Mizushima, N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol. 22, 132–139 (2010).
Sarkar, S. et al. Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat. Chem. Biol. 3, 331–338 (2007).
Eisenberg, T. et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314 (2009).
Hardie, D. G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8, 774–785 (2007).
Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Egan, D. F. et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 (2011).
Nishida, Y. et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461, 654–658 (2009).
Mizushima, N., Yoshimori, T. & Levine, B. Methods in mammalian autophagy research. Cell 140, 313–326 (2010).
Sou, Y. S. et al. The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol. Biol. Cell 19, 4762–4775 (2008).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Honda, S. et al. Ulk1-mediated Atg5-independent macroautophagy mediates elimination of mitochondria from embryonic reticulocytes. Nat. Commun. 5, 4004 (2014).
Kundu, M. et al. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112, 1493–1502 (2008).
Folmes, C. D. et al. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metabol. 14, 264–271 (2011).
Xu, X. et al. Mitochondrial regulation in pluripotent stem cells. Cell Metabol. 18, 325–332 (2013).
Li, F. et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol. Cell. Biol. 25, 6225–6234 (2005).
Fan, Y., Dickman, K. G. & Zong, W. X. Akt and c-Myc differentially activate cellular metabolic programs and prime cells to bioenergetic inhibition. J. Biol. Chem. 285, 7324–7333 (2010).
Hanna, J. et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462, 595–601 (2009).
Aune, U. L., Ruiz, L. & Kajimura, S. Isolation and differentiation of stromal vascular cells to beige/brite cells. J. Vis. Exp. 73, e50191 (2013).
Ma, T., Li, J. & Ding, S. Reprogramming of mouse fibroblasts into iPSCs. Protoc. Exch. http://dx.doi.org/10.1038/protex.2015.086 (2015).
Li, Y. et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 21, 196–204 (2011).
Acknowledgements
We thank Ding Laboratory members for suggestions and discussions. We thank J. Wong in the electron microscopy core at Gladstone Institutes for obtaining and analysing electron micrographs. We thank N. Mizushima (Tokyo Medical and Dental University, Tokyo, Japan) for permitting use of Atg5flox/flox mice and J. Debnath (The University of California, San Francisco, USA) for providing these mice with permission from N. Mizushima. We thank S. Shimizu (Tokyo Medical and Dental University, Tokyo, Japan) for providing plasmids encoding GFP–Rab9 and GFP–syntaxin7. We thank T. P. Bender (The University of Virginia, USA) for providing MIG-R1 and MIG-Cre. S.D. is supported by funding from NICHD, NHLBI, NEI/NIH, California Institute for Regenerative Medicine, and the Gladstone Institutes. J.L. was supported by a grant (No. 81372372) from the NSFC. This research was also supported by the ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences (No. XDA01040302).
Author information
Authors and Affiliations
Contributions
T.M., J.L. and Y.X. designed and performed the experiments, and analysed the data. C.Y., T.X., H.W., K.L., N.C., B.-m.N., S.-y.Z., S.X., K.L. and W.-g.W. performed the experiments and analysed the data. Y.W. provided materials and supervised the teratoma assay. K.-l.G. provided materials and advice. S.D. conceived the idea, designed the experiments and supervised the study. S.D., T.M., J.L. and Y.X. wrote the manuscript with inputs from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
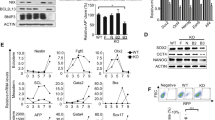
Supplementary Figure 2 Compounds screening for modulators of reprogramming.
(a) A schematic illustration of the chemical screening for iPSC reprogramming. (b) Scatter plot of all 1625 compounds. Secondary MEFs harboring Nanog-GFP reporter and doxycycline-inducible OSKM were seeded in 384-well plates at density of 1000 per well. The cells were treated with doxycycline (Dox) to induce reprogramming and cultured in mESC medium containing each individual chemical compound for 8 days. The cells were harvested on day 12 and subjected to GFP density detection. (c) Examples of morphology, GFP signal, and AP staining of reprogrammed cells in response to particular positive and negative hits. Scale bars represent 100 μm.
Supplementary Figure 3 AICAR, rapamycin or 3-MA modulated reprogramming in a manner independent of cell proliferation or transgene expression.
(a) Treatment with AICAR, rapamycin or 3-MA did not change the proliferation of reprogramming cells. MEFs transduced with OSKM were seeded in 6-well plates with density of 5 × 104 per well. Cells were cultured in mESC medium and treated with or without AICAR, rapamycin or 3-MA. On day 5, cell number was counted and shown. (b) 3-MA did not change the growth of MEFs. MEFs were treated with or without 3-MA, and cell number was counted at the indicated time points. (c,d) Treatment with AICAR, rapamycin or 3-MA did not enhance the transgene expression in reprogramming cells. MEFs were transduced with OSKM to induce reprogramming and then treated with or without AICAR or rapamycin. The expression of indicated transgenes was quantified using (c) qRT-PCR and (d) western blot. All values are mean ± s.d. (n = 3 independent experiments) and differences are significant for ∗∗P < 0.01 using student test. All representative images in panel d are shown for 3 independent experiments. Uncropped scans of blots are shown in Supplementary Fig. 7.
Supplementary Figure 4 Targeted genes and canonical autophagic flux was inhibited in knockdown and knockout assays.
(a–d) Targeted genes and canonical autophagic flux was inhibited by knockdown of Atg5 or Atg3. MEFs were transduced with Atg5 (a,b) or Atg3 (c,d) shRNAs and then were induced to reprogram by transduction with OSKM. The expression of indicated genes was analyzed in MEFs and reprogramming cells on day 3, using western blot. For analysis of LC3-II (b and d), cells were treated with 20 μM chloroquine for 6 h before harvest. (e,f) Deletion of Atg5 and block of canonical autophagic flux in Atg5−/− cells. Atg5−/− and Atg5flox/flox TTFs were constructed by expressing Cre-IRES-GFP and IRES-GFP in Atg5flox/flox TTFs respectively and then selected by GFP signal. Cells were induced to reprogram by overexpression of OSKM. The expression of indicated genes was analyzed in TTFs and reprogramming cells on day 3, using western blot. For analysis of LC3-II (f), cells were treated with 20 μM chloroquine for 6 h before harvest. All representative images in panels a–f are shown for 3 independent experiments. Uncropped scans of blots are shown in Supplementary Fig. 7.
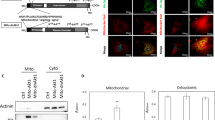
Supplementary Figure 5 Atg5-indepednt autophagy, not canonical autophagy, is induced by iPSC reprogramming.
(a) mRNA expression of autophagy related genes during OSKM-induced reprogramming of MEFs. The gene expression on day 0 was set to 1. (b) LC3-II was suppressed by OSKM-induced reprogramming. MEFs and reprogramming cells on day 3 were treated with or without 20 μM chloroquine (CQ) for 6 h before harvest. (c) Nanog+/GFP+ iPSC colonies were generated on day 14 of OSKM-induced reprogramming of Atg5−/− and Atg5flox/flox TTFs. Scale bars represent 100 μm. (d) No obvious autophagosomes were observed in MEFs. Representative MEF overexpressing GFP-Syntaxin7, Rab9, or LC3 was stained with Lamp2. Scale bars represent 20 μm. (e) Atg5 independent non-canonical rather than canonical autophagosomes were induced in MEFs undergoing OSKM-induced reprogramming. The percentage of cells with obvious GFP-Syntaxin7, Rab9, or LC3 puncta was quantified in MEFs and reprogramming cells on day 3. Data were derived from 30 random cells in each independent experiment. (f) ULK1 and Rab9 mRNA expressions were suppressed by their shRNAs in MEFs undergoing OSKM-induced reprogramming on day 3. (g) Deletion of ULK1 in ULK1−/− cells. ULK1−/− and ULK1flox/flox TTFs were induced to reprogram by overexpression of OSKM. ULK1 protein expression was analyzed in TTFs and reprogramming cells on day 3. (h) Overexpression of ULK1 enhanced OSKM or OSK-induced reprogramming of MEFs. The numbers of AP+ and Oct4-GFP+ colonies were counted on day 14 for OSKM-induction and on day 21 for OSK-induction. (i) Rab9 mRNA expression was steady during OSKM-induced reprogramming of MEFs. (j) Inhibition of AMPK blocked elevation of ULK1 phosphorylation at Ser317 in MEFs undergoing OSKM-induced reprogramming. Reprogramming cells were treated with or without 5 μM Dorsomorphin, and harvested on day 6. (k) Knockdown of AMPK prevented ULK1 phosphorylation at Ser317 in MEFs undergoing OSKM-induced reprogramming. Reprogramming cells were transfected with scramble (control) or AMPKα1&2 siRNAs, and harvested on day 6. All values are mean ± s.d. (n = 3 independent experiments) and differences are significant for ∗P < 0.05 and ∗∗P < 0.01 using student test. All representative images in panels b,c,d,g,j, and k are shown for 3 independent experiments. Uncropped scans of blots are shown in Supplementary Fig. 7.
Supplementary Figure 6 Inhibition of Atg5-independent autophagy blocks reprogramming-induced MET.
(a,b) The increase of E-cadherin expression upon iPSC reprogramming was delayed by transient treatment with 3-MA. MEFs were induced to reprogram by transduction with OSKM. Reprogramming cells were treated with or without 3-MA in the first 3 days. E-cadherin expression was analyzed using (a) qRT-PCR and (b) western blot at indicated time points. (c,d) ULK1 knockout prevented reprogramming cells from going through MET. ULK1−/− and ULK1flox/flox TTFs were transduced with OSKM to induce reprogramming. E-cadherin expression was analyzed using (c) qRT-PCR and (d) western blot at indicated time points. All values are mean ± s.d. (n = 3 independent experiments) and differences are significant for ∗P < 0.05 and ∗∗P < 0.01 using student test. All representative images in panels b and d are shown for 3 independent experiments. Uncropped scans of blots are shown in Supplementary Fig. 7.
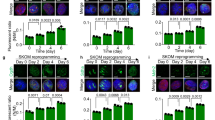
Supplementary Figure 7 Atg5-independent autophagy is responsible for removing mitochondria during iPSC reprogramming.
(a) A representative of autolysosomes (indicated by arrowhead) observed in Atg5−/− TTFs undergoing OSKM-induced reprogramming on day 3 using electron microscopy. Scale bar represents 0.5 μm. (b) Reduction of ERp72 protein in iPSC reprogramming is independent of Atg5, but dependent of lysosomal activity. Atg5−/− and Atg5flox/flox TTFs undergoing OSKM-induced reprogramming were treated with or without 20 μM chloroquine (CQ). ERp72 expression levels were analyzed using western blot in TTFs and reprogramming cells on day 3. (c–d) Atg5 knockout didn’t change the mitochondrial clearance in iPSC reprogramming. Atg5−/− and Atg5flox/flox TTFs were constructed by transducing Atg5flox/flox TTFs with Cre-pPGK-hygro and pPGK-hygro respectively and selected with hygromycin resistance. Atg5−/− and Atg5flox/flox TTFs were transduced with OSKM to induce reprogramming. (c) TTFs and reprogramming cells at indicated time points were stained with MitoTracker Green and analyzed using FACS. (d) Expression of indicated genes was analyzed using western blot in TTFs and reprogramming cells on day 3. Cells were treated with 20 μM chloroquine for 6 h before harvest. (e) The reduction of mitochondrial mass was observed during iPSC reprogramming. MEFs were transduced with OSKM and induced to reprogram. Reprogramming cells at indicated time points were stained with MitoTracker Red and analyzed using FACS. (f) The reprogramming-induced mitochondrial clearance was blocked by knockdown of ULK1 or Rab1, rather than knockdown of Atg5 or Atg3. MEFs were transduced with scramble or Atg5, Atg3, ULK1 or Rab9 shRNAs, and then were induced to reprogram by transduction with OSKM. Reprogramming cells were stained with MitoTracker Red on day 3, and analyzed with FACS. (g) Small-molecule modulators of autophagy change mitochondrial clearance in the reprogramming of Atg5−/− TTFs. Atg5−/− TTFs constructed by expressing Cre-IRES-GFP in Atg5flox/flox TTFs, were transduced with OSKM to induce reprogram, and treated with or without AICAR, rapamycin, SMER28 or 3-MA. TTFs and reprogramming cells on day 3 were stained with MitoTracker Red and analyzed in GFP+ cells using FASC. All representative images in panels b and d are shown for 3 independent experiments. Uncropped scans of blots are shown in Supplementary Fig. 7.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1305 kb)
Rights and permissions
About this article
Cite this article
Ma, T., Li, J., Xu, Y. et al. Atg5-independent autophagy regulates mitochondrial clearance and is essential for iPSC reprogramming. Nat Cell Biol 17, 1379–1387 (2015). https://doi.org/10.1038/ncb3256
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3256
This article is cited by
-
A fast chemical reprogramming system promotes cell identity transition through a diapause-like state
Nature Cell Biology (2023)
-
Atg5 knockout induces alternative autophagy via the downregulation of Akt expression
Toxicological Research (2023)
-
Mitochondrial Hydrogen Peroxide Activates PTEN and Inactivates Akt Leading to Autophagy Inhibition-Dependent Cell Death in Neuronal Models of Parkinson’s Disease
Molecular Neurobiology (2023)
-
Alternative autophagy: mechanisms and roles in different diseases
Cell Communication and Signaling (2022)
-
A degradative to secretory autophagy switch mediates mitochondria clearance in the absence of the mATG8-conjugation machinery
Nature Communications (2022)