Abstract
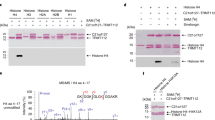
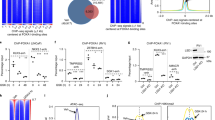
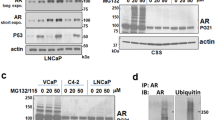
Posttranslational modifications of histones, such as methylation, regulate chromatin structure and gene expression1. Recently, lysine-specific demethylase 1 (LSD1)2, the first histone demethylase, was identified. LSD1 interacts with the androgen receptor and promotes androgen-dependent transcription of target genes by ligand-induced demethylation of mono- and dimethylated histone H3 at Lys 9 (H3K9)3 only. Here, we identify the Jumonji C (JMJC)4 domain-containing protein JMJD2C5,6 as the first histone tridemethylase regulating androgen receptor function. JMJD2C interacts with androgen receptor in vitro and in vivo. Assembly of ligand-bound androgen receptor and JMJD2C on androgen receptor-target genes results in demethylation of trimethyl H3K9 and in stimulation of androgen receptor-dependent transcription. Conversely, knockdown of JMJD2C inhibits androgen-induced removal of trimethyl H3K9, transcriptional activation and tumour cell proliferation. Importantly, JMJD2C colocalizes with androgen receptor and LSD1 in normal prostate and in prostate carcinomas. JMJD2C and LSD1 interact and both demethylases cooperatively stimulate androgen receptor-dependent gene transcription. In addition, androgen receptor, JMJD2C and LSD1 assemble on chromatin to remove methyl groups from mono, di and trimethylated H3K9. Thus, our data suggest that specific gene regulation requires the assembly and coordinate action of demethylases with distinct substrate specificities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Strahl, B. D. & Allis, C. D. The language of covalent histone modifications. Nature 403, 41–45 (2000).
Shi, Y. et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953 (2004).
Metzger, E. et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437, 436–439 (2005).
Tsukada, Y. I. et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature 435, 811–816 (2005).
Whetstine, J. R. et al. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell 125, 467–481 (2006).
Cloos, P. A. et al. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature 442, 307–311 (2006).
Shi, X. et al. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 442, 96–99 (2006).
Wysocka, J. et al. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature 442, 86–90 (2006).
Rosenfeld, M. G., Lunyak, V. V. & Glass, C. K. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 20, 1405–1428 (2006).
Yamane, K. et al. JHDM2A, a JmjC-containing H3K9 demethylase facilitates Transcription activation by androgen receptor. Cell 125, 483–495 (2006).
Trewick, S. C., McLaughlin, P. J. & Allshire, R. C. Methylation: lost in hydroxylation? EMBO Rep. 6, 315–320 (2005).
Fodor, B. D. et al. Jmjd2b antagonizes H3K9 trimethylation at pericentric heterochromatin in mammalian cells. Genes Dev. 20, 1557–1562 (2006).
Klose, R. J. et al. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature 442, 312–316 (2006).
Trojer, P. & Reinberg, D. Histone lysine demethylases and their impact on epigenetics. Cell 125, 213–217 (2006).
Kang, Z., Pirskanen, A., Janne, O. A. & Palvimo, J. J. Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J. Biol. Chem. 277, 48366–48371 (2002).
Metzger, E., Müller, J. M., Ferrari, S., Buettner, R. & Schüle, R. A novel inducible transactivation domain in the androgen receptor: implications for PRK in prostate cancer. EMBO J. 22, 270–280 (2003).
Gray, S. G. et al. Functional characterization of JMJD2A, a histone deacetylase- and retinoblastoma-binding protein. J. Biol. Chem. 280, 28507–28518 (2005).
Müller, J. M. et al. The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J. 21, 736–748 (2002).
Müller, J. M. et al. FHL2, a novel tissue-specific coactivator of the androgen receptor. EMBO J. 19, 359–369 (2000).
Shang, Y., Myers, M. & Brown, M. Formation of the androgen receptor transcription complex. Mol. Cell 9, 601–610 (2002).
Wiznerowicz, M. & Trono, D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 77, 8957–8961 (2003).
O'Neill, T. E., Roberge, M. & Bradbury, E. M. Nucleosome arrays inhibit both initiation and elongation of transcripts by bacteriophage T7 RNA polymerase. J. Mol. Biol. 223, 67–78 (1992).
Rigaut, G. et al. A generic protein purification method for protein complex characterization and proteome exploration. Nature Biotechnol. 17, 1030–1032 (1999).
Acknowledgements
We thank S. Gray for generously providing reagents. We thank K. Fischer, L. Walz, F. Klott and S. Vomstein for excellent technical assistance. We are obliged to M. Hoffmann and A. Schwentek from the sequencing core facility. We thank M. Follo for help with editing the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 747/P2, Schu 688/7-1, and Schu 688/9-1), the Dr. Hans Messner-Stiftung, and the Deutsche Krebshilfe (10-2019-Bu I) to R.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4 and S5 (PDF 194 kb)
Rights and permissions
About this article
Cite this article
Wissmann, M., Yin, N., Müller, J. et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol 9, 347–353 (2007). https://doi.org/10.1038/ncb1546
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1546
This article is cited by
-
KDM4C-mediated senescence defense is a targetable vulnerability in gastric cancer harboring TP53 mutations
Clinical Epigenetics (2023)
-
Emerging evidence that the mammalian sperm epigenome serves as a template for embryo development
Nature Communications (2023)
-
KDM4C silencing inhibits cell migration and enhances radiosensitivity by inducing CXCL2 transcription in hepatocellular carcinoma
Cell Death Discovery (2023)
-
Lysine-specific demethylase 1 as a corepressor of mineralocorticoid receptor
Hypertension Research (2022)
-
GASC1 promotes hepatocellular carcinoma progression by inhibiting the degradation of ROCK2
Cell Death & Disease (2021)