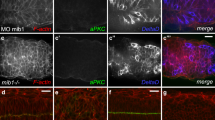
Abstract
Nrarp (Notch-regulated ankyrin repeat protein) is a small protein that has two ankyrin repeats1,2,3,4,5. Although Nrarp is known to be an inhibitory component of the Notch signalling pathway that operates in different developmental processes1,2,4,5, the in vivo roles of Nrarp have not been fully characterized. Here, we show that Nrarp is a positive regulator in the Wnt signalling pathway. In zebrafish, knockdown of Nrarp-a expression by an antisense morpholino oligonucleotide (MO) results in altered Wnt-signalling-dependent neural-crest-cell development. Nrarp stabilizes LEF1 protein, a pivotal transcription factor in the Wnt signalling cascade, by blocking LEF1 ubiquitination. In accordance with this, the knockdown phenotype of lef1 is similar to that of nrarp-a, at least in part, in its effect on the development of multiple tissues in zebrafish. Furthermore, activation of LEF1 does not affect Notch activity or vice versa. These findings reveal that Nrarp independently regulates canonical Wnt and Notch signalling by modulating LEF1 and Notch protein turnover, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Krebs, L. T., Deftos, M. L., Bevan, M. J. & Gridley, T. The Nrarp gene encodes an ankyrin-repeat protein that is transcriptionally regulated by the notch signalling pathway. Dev. Biol. 238, 110–119 (2001).
Lamar, E. et al. Nrarp is a novel intracellular component of the Notch signalling pathway. Genes Dev. 15, 1885–1899 (2001).
Topczewska, J. M., Topczewski, J., Szostak, A., Solnica-Krezel, L. & Hogan, B. L. Developmentally regulated expression of two members of the Nrarp family in zebrafish. Gene Expr. Patterns 3, 169–171 (2003).
Yun, T. J. & Bevan, M. J. Notch-regulated ankyrin-repeat protein inhibits Notch1 signalling: multiple Notch1 signalling pathways involved in T cell development. J. Immunol. 170, 5834–5841 (2003).
Pirot, P., van Grunsven, L. A., Marine, J. C., Huylebroeck, D. & Bellefroid, E. J. Direct regulation of the Nrarp gene promoter by the Notch signalling pathway. Biochem. Biophys. Res. Commun. 322, 526–534 (2004).
Moury, J. D. & Jacobson, A. G. The origins of neural crest cells in the axolotl. Dev. Biol. 141, 243–253 (1990).
Yanfeng, W., Saint-Jeannet, J. P. & Klein, P. S. Wnt-frizzled signalling in the induction and differentiation of the neural crest. Bioessays 25, 317–325 (2003).
Odenthal, J. & Nusslein-Volhard, C. fork head domain genes in zebrafish. Dev. Genes Evol. 208, 245–258 (1998).
Luo, R., An, M., Arduini, B. L. & Henion, P. D. Specific pan-neural crest expression of zebrafish Crestin throughout embryonic development. Dev. Dyn. 220, 169–174 (2001).
Dorsky, R. I., Moon, R. T. & Raible, D. W. Control of neural crest cell fate by the Wnt signalling pathway. Nature 396, 370–373 (1998).
Dorsky, R. I., Moon, R. T. & Raible, D. W. Environmental signals and cell fate specification in premigratory neural crest. Bioessays 22, 708–716 (2000).
Dorsky, R. I., Sheldahl, L. C. & Moon, R. T. A transgenic Lef1/β-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev. Biol. 241, 229–237 (2002).
Dorsky, R. I., Raible, D. W. & Moon, R. T. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 14, 158–162 (2000).
Peifer, M. & Polakis, P. Wnt signalling in oncogenesis and embryogenesis — a look outside the nucleus. Science 287, 1606–1609 (2000).
Ishitani, T., Ninomiya-Tsuji, J. & Matsumoto, K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/β-catenin signalling. Mol. Cell. Biol. 23, 1379–1389 (2003).
Dorsky, R. I. et al. Maternal and embryonic expression of zebrafish lef1. Mech. Dev. 86, 147–150 (1999).
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signalling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Holley, S. A., Geisler, R. & Nusslein-Volhard, C. Control of her1 expression during zebrafish somitogenesis by a δ-dependent oscillator and an independent wave-front activity. Genes Dev. 14, 1678–1690 (2000).
Itoh, M. et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signalling by Delta. Dev. Cell 4, 67–82 (2003).
Takke, C., Dornseifer, P., v Weizsacker, E. & Campos-Ortega, J. A. her4, a zebrafish homologue of the Drosophila neurogenic gene E(spl), is a target of NOTCH signalling. Development 126, 1811–1821 (1999).
Soriano, S. et al. Presenilin 1 negatively regulates β-catenin/T cell factor/lymphoid enhancer factor-1 signalling independently of β-amyloid precursor protein and notch processing. J. Cell Biol. 152, 785–794 (2001).
Axelrod, J. D., Matsuno, K., Artavanis-Tsakonas, S. & Perrimon, N. Interaction between Wingless and Notch signalling pathways mediated by dishevelled. Science 271, 1826–1832 (1996).
Ross, D. A. & Kadesch, T. The notch intracellular domain can function as a coactivator for LEF-1. Mol. Cell. Biol. 21, 7537–7544 (2001).
Foltz, D. R., Santiago, M. C., Berechid, B. E. & Nye, J. S. Glycogen synthase kinase-3β modulates notch signalling and stability. Curr. Biol. 12, 1006–1011 (2002).
Wettstein, D. A., Turner, D. L. & Kintner, C. The Xenopus homolog of Drosophila Suppressor of Hairless mediates Notch signalling during primary neurogenesis. Development 124, 693–702 (1997).
Kato, H. et al. Involvement of RBP-J in biological functions of mouse Notch1 and its derivatives. Development 124, 4133–4141 (1997).
Acknowledgements
We thank T. Honjo and C. Kintner for providing plasmid vectors; R.T. Moon for providing TOPdGFP fish; R.I. Dorsky for sharing information on lef1 splicing MO; H. Matsuo for maintaining fish; and K. Matsumoto laboratory members for helpful discussions. This research was supported by special grants from CREST and the Advanced Research on Cancer from the Ministry of Education, Culture and Science of Japan (K.M.), and JSPS Research Fellowship for Young Scientists (T.I.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figure S1, S2, S3 and S4 plus Supplementary material and methods (PDF 513 kb)
Rights and permissions
About this article
Cite this article
Ishitani, T., Matsumoto, K., Chitnis, A. et al. Nrarp functions to modulate neural-crest-cell differentiation by regulating LEF1 protein stability. Nat Cell Biol 7, 1106–1112 (2005). https://doi.org/10.1038/ncb1311
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1311
This article is cited by
-
NRARP displays either pro- or anti-tumoral roles in T-cell acute lymphoblastic leukemia depending on Notch and Wnt signaling
Oncogene (2020)
-
Integration of TGF-β-induced Smad signaling in the insulin-induced transcriptional response in endothelial cells
Scientific Reports (2019)
-
Cell competition corrects noisy Wnt morphogen gradients to achieve robust patterning in the zebrafish embryo
Nature Communications (2019)
-
Ubiquitin C-terminal hydrolase37 regulates Tcf7 DNA binding for the activation of Wnt signalling
Scientific Reports (2017)
-
NLK positively regulates Wnt/β-catenin signalling by phosphorylating LEF1 in neural progenitor cells
The EMBO Journal (2012)