Abstract
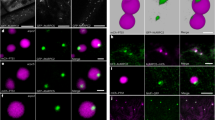
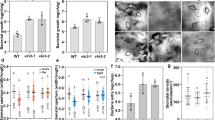
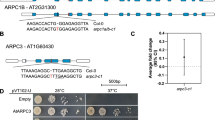
Formins are actin-organizing proteins that are involved in cytokinesis and cell polarity1. In the plant Arabidopsis thaliana, there are more than 20 formin homologues, all of which have unknown roles2. In this study, we characterize specific cellular and molecular functions of the Arabidopsis formin AtFH5. Despite the low identity of AtFH5 to yeast and mammalian formins, the AtFH5 protein interacts with the barbed end of actin filaments and nucleates actin-filament polymerization in vitro, as is the case in yeast and mammals. In vivo, the AtFH5–GFP fusion protein localizes to the cell plate, a plant-specific membranous component that is assembled at the plane of cell division. Consistent with these data, loss of function of atfh5 compromises cytokinesis in the seed endosperm. Furthermore, endogenous AtFH5 transcripts accumulate in the posterior pole of the endosperm and loss of function of atfh5 perturbs proper morphogenesis of the endosperm posterior pole. Although cytokinesis in animals, yeast and plants occurs through morphologically distinct mechanisms, our study finds that formin recruitment to sites of actin assembly is a common feature of cell division among eukaryotes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Evangelista, M., Zigmond, S. & Boone, C. Formins: signaling effectors for assembly and polarization of actin filaments. J. Cell Sci. 116, 2603–2611 (2003).
Deeks, M. J., Hussey, P. J. & Davies, B. Formins: intermediates in signal-transduction cascades that affect cytoskeletal reorganization. Trends Plant Sci. 7, 492–498 (2002).
Sørensen, M. B., Chaudhury, A. M., Robert, H., Bancharel, E. & Berger, F. Polycomb group genes control pattern formation in plant seed. Curr. Biol. 11, 277–281 (2001).
Cheung, A. Y. & Wu, H. M. Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell 16, 257–269 (2004).
Zigmond, S. H. Formin-induced nucleation of actin filaments. Curr. Opin. Cell Biol. 16, 1–7 (2004).
Evangelista, M., Pruyne, D., Amberg, D. C., Boone, C. & Bretscher, A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nature Cell Biol. 4, 32–41 (2002).
Kovar, D. R., Kuhn, J. R., Tichy, A. L. & Pollard, T. D. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 161, 875–887 (2003).
Deeks, M. J. & Hussey, P. J. Arp2/3 and 'the shape of things to come'. Curr. Opin. Plant Biol. 6, 561–567 (2003).
Blanchoin, L. et al. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASp/Scar proteins. Nature 404, 1007–1011 (2000).
Berger, F. Endosperm: the crossroad of seed development. Curr. Opin. Plant Biol. 6, 42–50 (2003).
Brown, R. C., Lemmon, B. E., Nguyen, H. & Olsen, O. A. Development of endosperm in Arabidopsis thaliana. Sex Plant Reprod. 12, 32–42 (1999).
Boisnard-Lorig, C. et al. Dynamic analyses of the expression of the HISTONE::YFP fusion protein in Arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell 13, 495–509 (2001).
Sørensen, M. B. et al. Cellularisation in the endosperm of Arabidopsis thaliana is coupled to mitosis and shares multiple components with cytokinesis. Development 129, 5567–5576 (2002).
Guitton, A. E. et al. Identification of new members of Fertilisation Independent Seed Polycomb Group pathway involved in the control of seed development in Arabidopsis thaliana. Development 131, 2971–2981 (2004).
Nguyen, H., Brown, R. C. & Lemmon, B. E. Patterns of cytoskeletal organization reflect distinct developmental domains in endosperm of Coronopus didymus (Brassicaceae). Intl J. Plant Sci. 162, 1–14 (2001).
Ketelaar, T., Anthony, R. G. & Hussey, P. J. Green fluorescent protein-mTalin causes defects in actin organization and cell expansion in Arabidopsis and inhibits actin depolymerizing factor's actin depolymerizing activity in vitro. Plant Physiol. 136, 3990–3998 (2004).
Huang, S., An, Y. Q., McDowell, J. M., McKinney, E. C. & Meagher, R. B. The Arabidopsis ACT11 actin gene is strongly expressed in tissues of the emerging inflorescence, pollen, and developing ovules. Plant Mol. Biol. 33, 125–139 (1997).
Shimada, A. et al. The core FH2 domain of diaphanous-related formins is an elongated actin binding protein that inhibits polymerization. Mol. Cell 13, 511–522 (2004).
Pruyne, D. et al. Role of formins in actin assembly: nucleation and barbed-end association. Science 297, 612–615 (2002).
Xu, Y. et al. Crystal structures of a Formin Homology-2 domain reveal a tethered dimer architecture. Cell 116, 711–723 (2004).
Verma, D. P. Cytokinesis and building of the cell plate in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 751–784 (2001).
Mayer, U. & Jürgens, G. Microtubule cytoskeleton: a track record. Curr. Opin. Plant Biol. 5, 494–501 (2002).
Robinson, D. N. & Spudich, J. A. Mechanics and regulation of cytokinesis. Curr. Opin. Cell Biol. 16, 182–188 (2004).
Staehelin, L. A. & Hepler, P. K. Cytokinesis in higher plants. Cell 84, 821–824 (1996).
Endle, M. C., Stoppin, V., Lambert, A. M. & Schmit, A. C. The growing cell plate of higher plants is a site of both actin assembly and vinculin-like antigen recruitment. Eur. J. Cell Biol. 77, 10–18 (1998).
Valster, A. H., Pierson, E. S., Valenta, R., Hepler, P. K. & Emons, A. Probing the plant actin cytoskeleton during cytokinesis and interphase by profilin microinjection. Plant Cell 9, 1815–1824 (1997).
Palevitz, B. & Hepler, P. K. The control of the plane of division during stomatal differentiation in Allium. II. Drug studies. Chromosoma 46, 327–341 (1974).
Schmit, A. C. & Lambert, A. M. Characterization and dynamics of cytoplasmic F-actin in higher plant endosperm cells during interphase, mitosis, and cytokinesis. J. Cell Biol. 105, 2157–2166 (1987).
Gu, X. & Verma, D. P. Dynamics of phragmoplastin in living cells during cell plate formation and uncoupling of cell elongation from the plane of cell division. Plant Cell 9, 157–169 (1997).
Huang, S., Blanchoin, L., Kovar, D. R. & Staiger, C. J. Arabidopsis capping protein (AtCP) is a heterodimer that regulates assembly at the barbed ends of actin filaments. J. Biol. Chem. 278, 44832–44842 (2003).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Jackson, D. Plant Pathology, Vol I: A Practical Approach (eds Gurr, S. J., McPherson, M. J. & Bowles, D. J.) 163–174 (IRL Press, Oxford, 1992).
Acknowledgements
We gratefully acknowledge the Institut National de la Recherche Agronomique Versailles and the SALK Institute for providing the insertion mutant lines; and the RIKEN Bioresource Center for prodiving the AtFH5 full-length cDNA clones. We thank C. Dumas for hosting F. B.'s team in his Reproduction et Développement des Plantes laboratory (Lyon, France). We thank A. Chaboud and S. Corneille for sharing Gateway plasmid constructs and S. Madi for valuable technical advices. We thank F.-Y. Bouget for his help in setting up a screen to identify phragmoplast-localized GFP-tagged proteins. This work was supported by the French Génoplante II programme (M.I.). The work was sponsored by the International Research Fellowship Program of the National Science Foundation (Grant INT 0301886; J.FG.), by the Fund for Scientific Research-Flanders (D.G. and D.V.D.), by a Marie Curie postdoctoral fellowship from the European Commission (M.B.S.), by the Institut National de la Recherche Agronomique (F.B., J.FG.) and by ATIPE from the Centre National de la Recherche Scientifique (C.G. and L.B.). F.B. is part of the European Molecular Biology Organisation Young Investigator Program. We thank D. Kovar, H. Higgs and T. Pollard for helpful suggestions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary figures S1, S2 and S3; supplementary methods (PDF 185 kb)
Rights and permissions
About this article
Cite this article
Ingouff, M., Gerald, J., Guérin, C. et al. Plant formin AtFH5 is an evolutionarily conserved actin nucleator involved in cytokinesis. Nat Cell Biol 7, 374–380 (2005). https://doi.org/10.1038/ncb1238
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1238
This article is cited by
-
Cellular dynamics of coenocytic endosperm development in Arabidopsis thaliana
Nature Plants (2023)
-
Transcriptome analysis reveals cell cycle-related transcripts as key determinants of varietal differences in seed size of Brassica juncea
Scientific Reports (2022)
-
The central cell nuclear position at the micropylar end is maintained by the balance of F-actin dynamics, but dispensable for karyogamy in Arabidopsis
Plant Reproduction (2015)
-
Rice LIM protein OsPLIM2a is involved in rice seed and tiller development
Molecular Breeding (2014)
-
Arabidopsis LIM proteins PLIM2a and PLIM2b regulate actin configuration during pollen tube growth
Biologia plantarum (2013)