Abstract
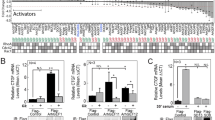
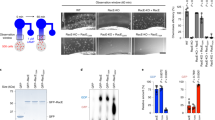
Gα13 stimulates the guanine nucleotide exchange factors (GEFs) for Rho, such as p115Rho-GEF1. Activated Rho induces numerous cellular responses, including actin polymerization, serum response element (SRE)-dependent gene transcription and transformation2. p115Rho-GEF contains a Regulator of G protein Signalling domain (RGS box) that confers GTPase activating protein (GAP) activity towards Gα12 and Gα13 (ref. 3). In contrast, classical RGS proteins (such as RGS16 and RGS4) exhibit RGS domain-dependent GAP activity on Gαi and Gαq, but not Gα12 or Gα13 (ref 4). Here, we show that RGS16 inhibits Gα13-mediated, RhoA-dependent reversal of stellation and SRE activation. The RGS16 amino terminus binds Gα13 directly, resulting in translocation of Gα13 to detergent-resistant membranes (DRMs) and reduced p115Rho-GEF binding. RGS4 does not bind Gα13 or attenuate Gα13-dependent responses, and neither RGS16 nor RGS4 affects Gα12-mediated signalling. These results elucidate a new mechanism whereby a classical RGS protein regulates Gα13-mediated signal transduction independently of the RGS box.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hart, M.J. et al. Direct stimulation of the guanine nucleotide exchange activity of p115RhoGEF by Gα13. Science 280, 2112–2114 (1998).
Sah, V.P., Seasholtz, T.M., Sagi, S.A. & Brown, J.H. The role of Rho in G protein-coupled receptor signal transduction. Annu. Rev. Pharm. Toxicol. 40, 459–489 (2000).
Fukuhara, S., Chikumi, H. & Gutkind, J.S. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene 20, 1661–1668 (2001).
Ross, E.M. & Wilkie, T.M. GTPase-activating proteins for heterotrimeric G proteins: regulator of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69, 795–827 (2000).
Schmitz, A.A.P., Govek, E.E., Boettner, B., & Van Aelst, L. Rho GTPases: signaling, migration, and invasion. Exp. Cell Res. 261, 1–12 (2000).
Seizinger, B.R., Gutkind, J.S. & Kley, N. The p53 tumor suppressor targets a novel regulator of G protein signaling. Proc. Natl. Acad. Sci. U.S.A. 94, 7868–7872 (1997).
Majumdar, M., Seasholtz, T.M., Buckmaster, C., Toksoz, D. & Brown, J.H. A Rho exchange factor mediates thrombin and Gα12-induced-induced cytoskeletal responses. J. Biol. Chem. 274, 26815–26821 (1999).
Arai, K. et al. Differential requirement of Gα12, Gα13, Gαq, and Gβγfor endothelin-1-induced JNK kinase and MAP kinase activation. Mol. Pharm. 63, 478–488 (2003).
Chen, C., Seow, K.T., Guo, K., Yaw, L.P. & Lin S.C. Characterization of a novel mammalian RGS protein that binds Gα and inhibits pheromone signaling in yeast. J. Biol. Chem. 272, 8679–8685 (1997).
Contos, J.J., Ishii, I. & Chun, J. Lysophosphatidic acid receptors. Mol. Pharmacol. 58, 1188–1209 (2000).
Schwartz, B.M. et al. Lysophosphatidic acids increase interleukin-8 expression in ovarian cancer cells. Gynecol. Oncol. 2, 291–300 (2001).
Druey, K.M., Blumer, K.J., Kang, V.H. & Kehrl, J.H. Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family. Nature 379, 742–746 (1996).
Booden, M.A., Siderovski, D.P. & Der, C.J. Leukemia-associated Rho guanine nucleotide exchange factor promotes Gαq-coupled activation of RhoA. Mol. Cell. Biol. 22, 4053–4061 (2002).
Krendel, M., Zenke, F. & Bokoch, G.M. Nucleotide exchange factor GEF-H1 mediates cross talk between microtubules and the actin cytoskeleton. Nature Cell. Biol. 4, 294–301 (2002).
Kozasa T. et al. p115RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science 280, 2109–2111 (1998).
Berman, D.M., Kozasa, T. & Gilman, A.G. The GTPase activating protein RGS4 stabilizes the transition state for nucleotide hydrolysis. J. Biol. Chem. 271, 27209–27212 (1996).
Popov, S., Yu, K., Kozasa, T. & Wilkie T.M. the regulators of G protein signaling (RGS) domains of RGS4, RGS10, and GAIP retain GTPase activating protein activity in vitro. Proc. Natl Acad. Sci. USA. 94, 7216–7220 (1997).
Derrien, A. et al. Src-mediated RGS16 tyrosine phosphorylation promotes RGS16 stability. J. Biol. Chem. 278, 16107–16116 (2003).
Battacharyya, R. & Wedegaertner, P.B. Gα13 requires palmitoylation for plasma membrane localization, Rho-dependent signaling, and promotion of p115RhoGEF membrane binding. J. Biol. Chem. 275, 14992–14999 (2000).
Hiol, A. et al. Palmitoylation regulates RGS16 function I. Mutation of amino terminal cysteine residues on RGS16 prevents its targeting to lipid rafts and palmitoylation of an internal cysteine residue. J. Biol. Chem. 278, 19301–19308 (2003).
Waheed, A.A. & Jones, T.L.Z. Hsp90 interactions and acylation target the G protein Gα12, but not Gα13 to lipid rafts. J. Biol. Chem. 277, 32409–32412 (2002).
Ingi, T. et al. Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J. Neurosci. 18, 7178–7188 (1998).
Bernstein, L.S., Grillo, A.A., Loranger, S.S. & Linder, M.E. RGS4 binds to membranes through an amphipathic alpha helix. J. Biol. Chem. 275, 18520–18526 (2000).
Druey, K.M., Uger, O., Caron, J.M., Chen, C.K., Backlund, P.S. & Jones, T.L. Amino terminal cysteine residues of RGS16 are required for palmitoylation and modulation of Gi and Gq mediated signaling. J. Biol. Chem. 274, 18836–18842 (1999).
Sullivan, B.M. et al. RGS4 and RGS2 bind coatomer and inhibit COPI association with Golgi membranes and intracellular transport. Mol. Biol. Cell 11, 3155–3168 (2000).
Wells, C.D., Jiang, X., Gutowski, S. & Sternweis, P.C. Functional characterization of p115RhoGEF. Meth. Enzymol. 345, 371–382 (2002).
Johnson, E.N. & Druey, K.M. Functional characterization of the G protein regulator RGS13. J. Biol. Chem. 277, 16768–16774 (2002).
Nagata, Y., Oda, M., Nakata, H., Shozaki, Y., Kozasa, T. & Todokoro, K. A novel regulator of G protein signaling bearing GAP activity for Gαi and Gαq in megakaryocytes. Blood 97, 3051–3060 (2001).
Kawamura, S., Miyamoto, S. & Brown, J.H. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of Erk translocation. J. Biol. Chem. 278, 31111–31117 (2003).
Brummelkamp, T.R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002).
Acknowledgements
We thank members of the Druey laboratory for discussions and D. Metcalfe for his support. This work was supported by the Division of Intra-Muro Research/National Institute of Health (E. N. J., K. M. D., A. A. W. and T. L. Z. J.), NIH grant GM36927 (J. H. B.) and an American HHeart Association, Scientist Development Grant (T. M. S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information, Fig. S1
Supplementary Information, Fig. S2 (PDF 218 kb)
Supplementary Information, Fig. S3
Supplementary Information, Fig. S4
Rights and permissions
About this article
Cite this article
Johnson, E., Seasholtz, T., Waheed, A. et al. RGS16 inhibits signalling through the Gα13–Rho axis. Nat Cell Biol 5, 1095–1103 (2003). https://doi.org/10.1038/ncb1065
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1065
This article is cited by
-
IL-33 priming and antigenic stimulation synergistically promote the transcription of proinflammatory cytokine and chemokine genes in human skin mast cells
BMC Genomics (2023)
-
RGS16 regulated by let-7c-5p promotes glioma progression by activating PI3K-AKT pathway
Frontiers of Medicine (2023)
-
Global transcriptomic changes in glomerular endothelial cells in mice with podocyte depletion and glomerulosclerosis
Cell Death & Disease (2021)
-
Gα13 regulates MEF2-dependent gene transcription in endothelial cells: role in angiogenesis
Angiogenesis (2009)
-
Rgs16
AfCS-Nature Molecule Pages (2006)