Abstract
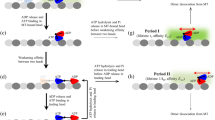
Kinesin is a molecular motor that moves processively1,2,3,4 by regular 8-nm steps along microtubules5,6,7,8,9,10,11. The processivity of this movement is explained by a hand-over-hand model in which the two heads of kinesin work in a coordinated manner. One head remains bound to the microtubule while the other steps from the αβ-tubulin dimer behind the attached head to the dimer in front. The overall movement is 8 nm per ATPase cycle9,10,11,12,13. To investigate elementary processes within the 8-nm step, we have developed a new assay that resolves nanometre displacements of single kinesin molecules with microsecond accuracy. Our data show that the 8-nm step can be resolved into fast and slow substeps, each corresponding to a displacement of ∼4 nm. The substeps are most probably generated by structural changes in one head of kinesin, leading to rectified forward thermal motions of the partner head14. It is also possible that the kinesin steps along the 4-nm repeat of tubulin monomers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vale, R. D., Reese, T. S. & Sheetz, M. P. Cell 42, 39–50 (1985).
Howard, J., Hudspeth, A. J. & Vale, R. D. Nature 342, 154–158 (1989).
Gilbert, S. P., Webb, M. R., Brune, M. & Johnson, K. A. Nature 373, 671–676 (1995).
Hackney, D. D. Nature 377, 448–450 (1995).
Higuchi, H., Muto, E., Inoue, Y. & Yanagida, T. Proc. Natl Acad. Sci. USA 94, 4395–4400 (1997).
Crevel, I., Carter, N., Schliwa, M. & Cross, R. EMBO J. 18, 5863–5872 (1999).
Coppin, C. M., Finer, J. T., Spudich, J. A. & Vale, R. D. Proc. Natl Acad. Sci. USA 93, 1913–1917 (1996).
Svoboda, K., Schmidt, C. F., Schnapp, B. J. & Block, S. M. Nature 365, 721–727 (1993).
Kojima, H., Muto, E., Higuchi, H. & Yanagida, T. Biophys. J. 73, 2012–2022 (1997).
Schnitzer, M. J. & Block, S. M. Nature 388, 386–390 (1997).
Visscher, K., Schnitzer, M. J. & Block, S. M. Nature 400, 184–189 (1999).
Hua, W., Young, E. C., Fleming, M. L. & Gelles, J. Nature 388, 390–393 (1997).
Coy, D. L., Wagenbach, M. & Howard, J. J. Biol. Chem. 274, 3667–3671 (1999).
Rice, S. et al. Nature 402, 778–784 (1999).
Allersma, M. W., Gittes, F., deCastro, M. J., Stewart, R. J. & Schmidt, C. F. Biophys. J. 74, 1074–1085 (1998).
Svoboda, K. & Block, S. M. Cell 77, 773–784 (1994).
Meyhofer, E. & Howard, J. Proc. Natl Acad. Sci. USA 92, 574–578 (1995).
Kudo, S., Magariyama, Y. & Aizawa, S. Nature 346, 677–680 (1990).
Hirose, K., Lockhart, A., Cross, R. A. & Amos, L. A. Proc. Natl Acad. Sci. USA 93, 9539–9544 (1996).
Walker, R. A. Proc. Natl Acad. Sci. USA 92, 5960–5964 (1995).
Larcher, J. C., Boucher, D., Lazereg, S., Gros, F. & Denoulet, P. J. Biol. Chem. 271, 22117–22124 (1996).
Tucker, C. & Goldstein, L. S. B. J. Biol. Chem. 272, 9481–9488 (1997).
Nogales, E., Wolf, S. G. & Downing, K. H. Nature 391, 199–203 (1998).
Nogales, E., Whittaker, M., Milligan, R. A. & Downing, K. H. Cell 96, 79–88 (1999).
Nishiyama, M., Higuchi, H., Muto, E., Inoue, Y. & Yanagida, T. Biophys. J. 78, 122A (2000).
Acknowledgements
We thank K. Kitamura and A. Ishijima for technical suggestions, Y. Ishii and other colleagues of Single Molecule Processes Project, JST and Osaka University for valuable discussions, and H. Kojima, Y. E. Goldman, F. Brozovich, Y. Ishii Jr, J. West and S. A. Endow for critically reading the manuscript. This work was partially supported by JSPS Research Fellowships for Young Scientists (M.N.), the Japan Ministry of Education, Science, Sport and Culture (H.H.), and by Yanagida BioMotron Project, ERATO, JST. A preliminary report of this investigation has been presented previously25.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishiyama, M., Muto, E., Inoue, Y. et al. Substeps within the 8-nm step of the ATPase cycle of single kinesin molecules. Nat Cell Biol 3, 425–428 (2001). https://doi.org/10.1038/35070116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35070116
This article is cited by
-
Planar photonic chips with tailored angular transmission for high-contrast-imaging devices
Nature Communications (2021)
-
A modified active Brownian dynamics model using asymmetric energy conversion and its application to the molecular motor system
Journal of Biological Physics (2013)
-
Detection of Steps in Single Molecule Data
Cellular and Molecular Bioengineering (2012)
-
Strain through the neck linker ensures processive runs: a DNA-kinesin hybrid nanomachine study
The EMBO Journal (2010)
-
Kinesin velocity increases with the number of motors pulling against viscoelastic drag
European Biophysics Journal (2010)