Abstract
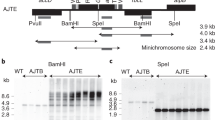
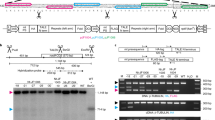
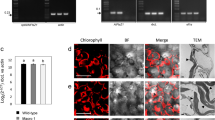
Genetic engineering of higher plant plastids typically involves stable introduction of antibiotic resistance genes as selection markers. Even though chloroplast genes are maternally inherited in most crops1, the possibility of marker transfer to wild relatives2 or microorganisms3 cannot be completely excluded. Furthermore, marker expression can be a substantial metabolic drain4. Therefore, efficient methods for complete marker removal from plastid transformants are necessary. One method to remove the selection gene from higher plant plastids is based on loop-out recombination5, a process difficult to control because selection of homoplastomic transformants is unpredictable. Another method uses the CRE/lox system6,7, but requires additional retransformation and sexual crossing for introduction and subsequent removal of the CRE recombinase. Here we describe the generation of marker-free chloroplast transformants in tobacco using the reconstitution of wild-type pigmentation8 in combination with plastid transformation vectors, which prevent stable integration of the kanamycin selection marker9. One benefit of a procedure using mutants is that marker-free plastid transformants can be produced directly in the first generation (T0) without retransformation or crossing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Maliga, P., Carrer, H., Kanevski, I., Staub, J. & Svab, Z. Plastid engineering in land plants: a conservative genome is open to change. Phil. Trans. R. Soc. Lond. B 342, 203–208 (1993).
Daniell, H. Molecular strategies for gene containment in transgenic crops. Nat. Biotechnol. 20, 581–586 (2002).
Kay, E., Vogel, T.M., Bertolla, F., Nalin, R. & Simonet, P. In situ transfer of antibiotic resistance genes from transgenic (transplastomic) tobacco plants to bacteria. Appl. Environ. Microbiol. 68, 3345–3351 (2002).
Kuroda, H. & Maliga, P. Sequences downstream of the translation initiation codon are important determinants of translation efficiency in chloroplasts. Plant Physiol. 125, 430–436 (2001).
Iamtham, S. & Day, A. Removal of antibiotic resistance genes from transgenic tobacco plastids. Nat. Biotechnol. 18, 1172–1176 (2000).
Corneille, S., Lutz, K., Svab, Z. & Maliga, P. Efficient elimination of selectable marker genes from the plastid genome by the CRE-lox site-specific recombination system. Plant J. 27, 171–178 (2001).
Hajdukiewicz, P.T., Gilbertson, L. & Staub, J.M. Multiple pathways for Cre/lox-mediated recombination in plastids. Plant J. 27, 161–170 (2001).
Klaus, S.M., Huang, F.C., Eibl, C., Koop, H.U. & Golds, T.J. Rapid and proven production of transplastomic tobacco plants by restoration of pigmentation and photosynthesis. Plant J. 35, 811–821 (2003).
Huang, F.C. et al. Efficient plastid transformation in tobacco using the aphA-6 gene and kanamycin selection. Mol. Genet. Genomics 268, 19–27 (2002).
Staub, J.M. & Maliga, P. Expression of a chimeric uidA gene indicates that polycistronic mRNAs are efficiently translated in tobacco plastids. Plant J. 7, 845–848 (1995).
Maliga, P. Progress towards commercialization of plastid transformation technology. Trends Biotechnol 21, 20–28 (2003).
Boynton, J.E. et al. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 240, 1534–1537 (1988).
Staub, J.M. & Maliga, P. Extrachromosomal elements in tobacco plastids. Proc. Natl. Acad. Sci. USA 91, 7468–7472 (1994).
Staub, J.M. & Maliga, P. Marker rescue from the Nicotiana tabacum plastid genome using a plastid/Escherichia coli shuttle vector. Mol. Gen. Genet. 249, 37–42 (1995).
Sidorov, V.A. et al. Stable chloroplast transformation in potato: use of green fluorescent protein as a plastid marker. Plant J. 19, 209–216 (1999).
Ruf, S., Hermann, M., Berger, I.J., Carrer, H. & Bock, R. Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat. Biotechnol. 19, 870–875 (2001).
Eibl, C. et al. In vivo analysis of plastid psbA, rbcL and rpl32 UTR elements by chloroplast transformation: tobacco plastid gene expression is controlled by modulation of transcript levels and translation efficiency. Plant J. 19, 333–345 (1999).
Svab, Z., Hajdukiewicz, P. & Maliga, P. Stable transformation of plastids in higher plants. Proc. Natl. Acad. Sci. USA 87, 8526–8530 (1990).
Gamborg, O.L., Miller, R.A. & Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50, 151–158 (1968).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497 (1962).
Jefferson, R.A., Bevan, M. & Kavanagh, T. The use of the Escherichia coli beta-glucuronidase as a gene fusion marker for studies of gene expression in higher plants. Biochem. Soc. Trans. 15, 17–18 (1987).
Acknowledgements
The authors thank Christian Eibl and Cornelia Stettner for helpful discussions and careful reading of the manuscript. Expert technical assistance was provided by Carolin Adams, Angela Alkofer and Simin Erschadi. This work was supported in part by Bayerische Forschungsstiftung (grant no. 356/99) and Bayerisches Staatsministerium für Wirtschaft, Verkehr und Technologie (grant no. 3600 - VIII/1e-15520).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All authors are employees of ICON Genetics, a private biotechnology company.
Rights and permissions
About this article
Cite this article
Klaus, S., Huang, FC., Golds, T. et al. Generation of marker-free plastid transformants using a transiently cointegrated selection gene. Nat Biotechnol 22, 225–229 (2004). https://doi.org/10.1038/nbt933
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt933
This article is cited by
-
Engineering the plastid and mitochondrial genomes of flowering plants
Nature Plants (2022)
-
A novel non-antibiotic selectable marker GASA6 for plant transformation
Plant Cell, Tissue and Organ Culture (PCTOC) (2022)
-
The fate of extrachromosomal DNAs in the progeny of plastid-transformed tobacco plants
Plant Biotechnology Reports (2015)