Abstract
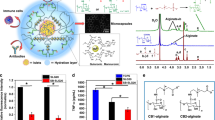
We have developed a gel entrapment process for the entrapment and growth of mu-rine hybridoma cells. This entrapment process takes advantage of the calcium-mediated gelation of sodium alginate. Mu-rine hybridoma cells entrapped within calcium alginate gel microbeads were shown to proliferate to high cell density while maintaining high cell viability. The rate of monoclonal antibody (Mab) production by calcium alginate gel-entrapped murine hybridoma cells was identical to control suspension cultures indicating that alginate gels do not effect the production or restrict diffusion of murine IgG into the medium. Gel-entrapped hybridoma cells cultured in 1.0L bioreactors under dissolved oxygen control grew to tenfold higher viable cell densities than cells grown in conventional suspension culture. The resultant concentration of Mab in medium from cultures of gel-entrapped hybridoma cells was concomitantly increased by a factor of ten.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Mattiasson, B. 1983. Immobilization methods, p. 4–19. In: Immobilized Cells and Organelles, Vol. 1. Mattiasson, B. (Ed.) CRC Press, Inc., Boca Raton, Florida.
Nilsson, K., Scheirer, W., Merten, O.W. Ostberg, L., Liehl, E., Katinger, D., and Mosbach, K. 1983 Entrapment of animal cells for production of monoclonal antibodies and other biomolecules. Nature 302:629–630.
Nilsson, K. and Mosbach, K. 1980 Preparation of immobilized animal cells. FEBS Lett. 118:145–150.
Nilsson, K., et al. 1983. A general method for the immobilization of cells with preserved viability. Eur. J. Appl. Microbiol. Biotechnol. 17:319–326.
Schierer, W., Nilsson, K., Merten, O.W. Katinger, H.W.D., and Mosbach, K. 1980. Entrapment of animal cells for the production of biomolecules such as monoclonal antibodies. Devel. Biol. Standard 55:155–161.
Pilwat, G., Washausen, P., Llein, K. and Zimmerman, L. 1980. Immobilization of human red blood cells. Biochem. Biophys. Biol. Virol. 35:352.
Kupchik, H.Z., Langer, R.S., Habern, S. El Deriny and O'Brien, M. 1983. A new method for the three-dimensional in vitro growth of human cancer cells. Exp. Cell. Res. 147:454–460.
Lim, F. and Sun, A.M. 1980. Microencapsulated islets as bioartincial endocrine pancreas. Science 210:908–910.
Jarvis, A.P., Grdina, T.A. and Sullivan, M.F. 1986. Cell growth and hemoglobin synthesis in murine erythroleukenic cells propagated in high density microcapsule culture. In Vitro 22:589–596.
McNeely and Pettitt, D.J. 1973. Industrial Gums, p. 49–82. Whistler, R. L. and BeMiller, J. N. (Eds.) Academic Press, Inc., N.Y., N.Y.
Iijima, S., Mano, T., Taniguchi, M. and Kobayashi, T. 1988. Immobilization of hybridoma cells with alginate and methane polymer and improved monoclonal antibody production. Appl. Microbiol. Biotechnol. 28:572–576.
Shirai, Y., Sasaki, R., Hashimoto, K., Kawahara, H., Hitami, K., and Chiba, H., 1988. Continuous production of erythropoietin with immobilized animal cells. Appl. Microbiol. Biotechnol. 29:544–549.
Familletti, P.C. and Fredericks, J.E. 1988. Techniques formammlian cell immobilization. Nature Biotechnology 6:41–44.
Tanaka, H., Masatoni, M. and Vellky, I.A. 1984. Diffusion characteristics of substrates in calcium alginate gel beads. Biotechnol. Bioeng. 26:56–58.
Cheetham, P.S. Blunt, K.W. and Burke, C. 1979. Physical studies on cell immobilization using calcium alginate gels. Biotechnol. Bioeng. 21:2155–2168.
Brodelius, P. and Mosbach, K. 1982. Immobilized plant cells. Adv. Applied Microbiology. 28:1–26.
Tanaka, H., Kurosawa, H., Kokutata, E. and Vellky, I.A. 1984. Preparation of immobilized glucoamylase using Ca-alginate gel coated with partially quaternized poly(ethyleneimine). Biotechnol. Bioeng. 26:1393–1394.
Roscoe, J.P. and Owsianka, A.M. 1982. Alginate: A reversible semisolid medium for investigating cell transformation. Br. J. Cancer. 46:965–969.
Glacken, M.W. Fleischaker, R.J. and Siskey, A.J. 1983. Large-scale production of mammalian cells and their products: Engineering principles and barriers to scale-up. Annals NY Acad. Sci. 413:355–372.
Reuveny, S., Velez, D., Miller, L., and MacMillan, J.D. 1986. Comparison of cell propagation methods for their effect on monoclonal antibody yield in fermentors. J. Immunol. Methods. 86:61–69.
Velez, D., Reuveny, S., Miller, L., and MacMillan, J.D. 1987. Effect of feeding rate on monoclonal antibody production in a modified perfusion-fed fermentor. J. Immunol. Methods. 102:275–278.
Fazekas de St. Groth. 1983. Automated production of monoclonal antibodies in a cytostat. J. Immunol. Methods. 57:121–136.
Shirai, Y., Hashimoto, K., Yamaji, H. and Tokashiki, M. 1987. Continuous production of monoclonal antibody with immobilized hybridoma cells in an expanded bed fermentor. Appl. Microbiol. Biotechnol. 26:495–499.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sinacore, M., Creswick, B. & Buehler, R. Entrapment and Growth of Murine Hybridoma Cells in Calcium Alginate Gel Microbeads. Nat Biotechnol 7, 1275–1279 (1989). https://doi.org/10.1038/nbt1289-1275
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt1289-1275