Abstract
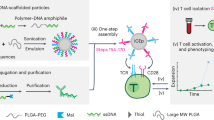
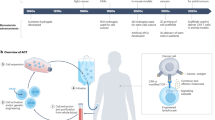
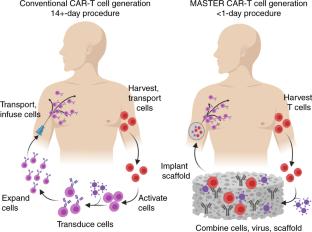
Despite their clinical success, chimeric antigen receptor (CAR)-T cell therapies for B cell malignancies are limited by lengthy, costly and labor-intensive ex vivo manufacturing procedures that might lead to cell products with heterogeneous composition. Here we describe an implantable Multifunctional Alginate Scaffold for T Cell Engineering and Release (MASTER) that streamlines in vivo CAR-T cell manufacturing and reduces processing time to a single day. When seeded with human peripheral blood mononuclear cells and CD19-encoding retroviral particles, MASTER provides the appropriate interface for viral vector-mediated gene transfer and, after subcutaneous implantation, mediates the release of functional CAR-T cells in mice. We further demonstrate that in vivo-generated CAR-T cells enter the bloodstream and control distal tumor growth in a mouse xenograft model of lymphoma, showing greater persistence than conventional CAR-T cells. MASTER promises to transform CAR-T cell therapy by fast-tracking manufacture and potentially reducing the complexity and resources needed for provision of this type of therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its extended data and supplementary information files. Source data are provided with this paper.
References
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Enblad, G. et al. A phase I/IIa trial using CD19-targeted third-generation CAR T cells for lymphoma and leukemia. Clin. Cancer Res. 24, 6185–6194 (2018).
Ramos, C. A., Savoldo, B. & Dotti, G. CD19-CAR trials. Cancer J. 20, 112–118 (2014).
Prasad, V. Tisagenlecleucel—the first approved CAR-T-cell therapy: implications for payers and policy makers. Nat. Rev. Clin. Oncol. 15, 11–12 (2018).
Sharma, P., King, G. T., Shinde, S. S., Purev, E. & Jimeno, A. Axicabtagene ciloleucel for the treatment of relapsed/refractory B-cell non-Hodgkin’s lymphomas. Drugs Today 54, 187–198 (2018).
Tang, J., Hubbard-Lucey, V. M., Pearce, L., O’Donnell-Tormey, J. & Shalabi, A. The global landscape of cancer cell therapy. Nat. Rev. Drug Discov. 17, 465–466 (2018).
Bach, P. B. National coverage analysis of CAR-T therapies—policy, evidence, and payment. N. Engl. J. Med. 379, 1396–1398 (2018).
Hernandez, I. Analysis determines true cost for CAR T-cell therapy. Healio https://www.healio.com/news/hematology-oncology/20180426/analysis-determines-true-cost-for-car-tcell-therapy (2018).
Leyfman, Y. Chimeric antigen receptors: unleashing a new age of anti-cancer therapy. Cancer Cell Int. 18, 182 (2018).
Caffrey, M. With approval of CAR T-cell therapy comes the next challenge: payer coverage. Am. J. Manag. Care 24, SP35–SP36 (2018).
Jackson, H. J., Rafiq, S. & Brentjens, R. J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 13, 370–383 (2016).
Shah, N. N. & Fry, T. J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 16, 372–385 (2019).
Park, J. H. et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 378, 449–459 (2018).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Ghassemi, S. et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol. Res. 6, 1100–1109 (2018).
Xu, Y. & Dotti, G. Selection bias: maintaining less-differentiated T cells for adoptive immunotherapy. J. Clin, Invest. 126, 35–37 (2016).
Gattinoni, L., Klebanoff, C. A. & Restifo, N. P. Paths to stemness: building the ultimate antitumour T cell. Nat. Rev. Cancer 12, 671–684 (2012).
Mock, U. et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS prodigy. Cytotherapy 18, 1002–1011 (2016).
Lu, T. L. et al. A rapid cell expansion process for production of engineered autologous CAR-T cell therapies. Hum. Gene Ther. Methods 27, 209–218 (2016).
Depil, S., Duchateau, P., Grupp, S. A., Mufti, G. & Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19, 185–199 (2020).
Kim, D. W. & Cho, J.-Y. Recent advances in allogeneic CAR-T cells. Biomolecules 10, 263 (2020).
Smith, T. T. et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 12, 813–820 (2017).
Agarwal, S., Weidner, T., Thalheimer, F. B. & Buchholz, C. J. In vivo generated human CAR T cells eradicate tumor cells. Oncoimmunology 8, e1671761 (2019).
Dautzenberg, I. J. C., Rabelink, M. J. W. E. & Hoeben, R. C. The stability of envelope-pseudotyped lentiviral vectors. Gene Ther. 28, 89–104 (2021).
Rosenblum, D., Joshi, N., Tao, W., Karp, J. M. & Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 9, 1410 (2018).
Lee, K. Y. & Mooney, D. J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106–126 (2012).
Andersen, T., Auk-Emblem, P. & Dornish, M. 3D cell culture in alginate hydrogels. Microarrays (Basel) 4, 133–161 (2015).
Hwang, C. M. et al. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication 2, 035003 (2010).
Agarwalla, P. et al. Scaffold‐mediated static transduction of T cells for CAR‐T cell therapy. Adv. Healthc. Mater. 9, e2000275 (2020).
Savina, I. N., Ingavle, G. C., Cundy, A. B. & Mikhalovsky, S. V. A simple method for the production of large volume 3D macroporous hydrogels for advanced biotechnological, medical and environmental applications. Sci. Rep. 6, 21154 (2016).
Shapiro, L. & Cohen, S. Novel alginate sponges for cell culture and transplantation. Biomaterials 18, 583–590 (1997).
Brudno, Y. et al. In vivo targeting through click chemistry. ChemMedChem 10, 617–620 (2015).
Cheung, A. S., Zhang, D. K. Y., Koshy, S. T. & Mooney, D. J. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat. Biotechnol. 36, 160–169 (2018).
Stephan, S. B. et al. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 33, 97–101 (2015).
Moody, C. T., Palvai, S. & Brudno, Y. Click cross-linking improves retention and targeting of refillable alginate depots. Acta Biomater. 112, 112–121 (2020).
Savoldo, B. et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 121, 1822–1826 (2011).
Stock, S. et al. Influence of retronectin-mediated T-cell activation on expansion and phenotype of CD19-specific chimeric antigen receptor T cells. Hum. Gene Ther. 29, 1167–1182 (2018).
Hori, Y., Winans, A. M. & Irvine, D. J. Modular injectable matrices based on alginate solution/microsphere mixtures that gel in situ and co-deliver immunomodulatory factors. Acta Biomater. 5, 969–982 (2009).
Diaconu, I. et al. Inducible caspase-9 selectively modulates the toxicities of CD19-specific chimeric antigen receptor-modified T cells. Mol. Ther. 25, 580–592 (2017).
Xu, Y. et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123, 3750–3759 (2014).
Ramos, C. A. et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J. Clin. Invest. 127, 3462–3471 (2017).
Ramos, C. A. et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J. Clin. Invest. 126, 2588–2596 (2016).
Vu, H. N., Ramsey, J. D. & Pack, D. W. Engineering of a stable retroviral gene delivery vector by directed evolution. Mol. Ther 16, 308–314 (2008).
Tay, R. E., Richardson, E. K. & Toh, H. C. Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms. Cancer Gene Ther. 28, 5–17 (2021).
Wen, H. et al. Preclinical safety evaluation of chimeric antigen receptor-modified T cells against CD19 in NSG mice. Ann. Transl. Med. 7, 735 (2019).
Zhao, Z. et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28, 415–428 (2015).
McLellan, A. D. & Ali Hosseini Rad, S. M. Chimeric antigen receptor T cell persistence and memory cell formation. Immunol. Cell Biol. 97, 664–674 (2019).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021).
Majzner, R. G. & Mackall, C. L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8, 1219–1226 (2018).
Liu, L. et al. Enhanced CAR-T activity against established tumors by polarizing human T cells to secrete interleukin-9. Nat. Commun. 11, 5902 (2020).
Gostick, J. et al. OpenPNM: a pore network modeling package. Comput. Sci. Eng. 18, 60–74 (2016).
Vera, J. et al. T lymphocytes redirected against the κ light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 108, 3890–3897 (2006).
Xu, Y. et al. Glycolysis determines dichotomous regulation of T cell subsets in hypoxia. J. Clin. Invest. 126, 2678–2688 (2016).
Ramos, C. A. et al. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin lymphoma. J. Clin. Oncol. 38, 3794–3804 (2020).
Acknowledgements
This work was supported by the North Carolina Biotechnology Center Flash Grant 2019-FLG-3812; by the National Center for Advancing Translational Sciences and the National Institutes of Health through grant awards R37-CA260223, UL1-TR002489, R01-CA193140, R21-CA229938, T32-CA196589 and R25-NS094093; and by start-up funds provided by the University of North Carolina at Chapel Hill, North Carolina State University at Raleigh, the Lineberger Cancer Center and the Ross M. Lampe Endowed Chair. We thank the North Carolina State University College of Veterinary Medicine staff for proper care of animals used in experiments and valuable resources on training. We also thank the North Carolina State University flow cytometry core and J. Mohammed for training and guidance on flow cytometry analysis. We are grateful to T. Xianming for assistance with power analysis and to C. Clifford for evaluating histology samples. SEM and X-ray CT images were taken at the Analytical Instrumentation Facility at North Carolina State University, which is supported by the State of North Carolina and the National Science Foundation (award ECCS-1542015). The Analytical Instrumentation Facility is a member of the North Carolina Research Triangle Nanotechnology Network, a site in the National Nanotechnology Coordinated Infrastructure. The authors acknowledge the use of the Cellular and Molecular Imaging Facility at North Carolina State University, which is supported by the State of North Carolina and the National Science Foundation. Schematics were created with BioRender.
Author information
Authors and Affiliations
Contributions
P.A. conceived of the study, designed and performed experiments, analyzed data and wrote the paper. Y.B. conceived of the study, analyzed data and wrote the paper. E.A.O., S.A., K.F. and A.J. prepared experimental materials and performed experiments. F.S.L. and G.D. contributed to the design of experiments and to writing and editing the paper. All authors discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
P.A., G.D. and Y.B. are inventors on patents related to the use of biomaterials for generation of CAR-T cell therapeutics. Y.B. receives an industry-sponsored research grant related to CAR-T cell therapeutic technology (unrelated to this work). G.D. is a paid consultant for Bellicum Pharmaceuticals, Tessa Therapeutics and Catamaran. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks Jeffrey Hubbell, Prasad Adusumilli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Quantitative characterization of MASTER scaffold structure.
A) Scanned volume of MASTER (left) with a colored plane indicating the cross-section seen on the right. In the cross sections a brighter value indicates a higher density (scaffolds) and a darker value a lower density (air porosity). B) Relative frequency of pores of different dimensions. C) The aspect ratio of the pores showing most of the pores has an oblong shape. An aspect ratio of 1 corresponds to a sphere and close to 0 corresponds to a flat plane or stick. D) The surface area as a function of volume plotted. The total surface area inside of MASTER is roughly 810 mm2 E) Connectivity of sample showing most of the pores are connected to 0-3 other pores with a very few pores (around 6%) have more connections than 9.
Extended Data Fig. 2 Confocal images of GFP-expressing T cells within MASTER scaffold.
3D confocal micrograph showing distribution of GFP + T cells in AF647 labeled MASTER at 10X (A) and 40X (B) magnification. This experiment was repeated twice independently with similar results.
Extended Data Fig. 3 IL-2 loaded onto MASTER released in a sustained manner over five days in vitro and retained its bioactivity.
(A) Cumulative release of IL-2 from MASTER as quantified by ELISA assay. Data represent mean ± SD of three independent samples (B) Bioactivity of IL2 released at 24 hours as assessed by proliferation of CFSE stained T cells.
Extended Data Fig. 4 IL-2 promotes lymphocyte proliferation, but not transduction efficiency of MASTER.
(A) MASTER and MASTER without IL-2 was seeded with PBMCs and virus and number of cells were counted 5 days post transduction. *p < 0.05, two tailed unpaired t test, (B) CAR.19 expression in T cells 72 h post transduction. *p < 0.05, two-tailed unpaired t test. Data represent median of n = 3 biologically independent samples.
Extended Data Fig. 5 MASTER functions as an efficient T cell-release system.
A) Schematic of in vitro release study. B) Percent of cells released from scaffold. Data in represent mean ± SD of n = 3 independent samples.
Extended Data Fig. 6 Biocompatibility of MASTER and its components.
Representative images of H&E-stained sections of five major organs and implantation site four weeks after subcutaneous implant of MASTER, MASTER + mouse PBMCs + GFP-encoding gamma retrovirus and untreated controls in C57Bl6/J immunocompetent mice. Data is representative of three biologically independent animals.
Extended Data Fig. 7 MASTER loaded with PBMCs and retrovirus does not transduce host cells.
A) In vitro transwell model mimicking the in vivo system. B) GFP expression in fibroblast cells seeded on the bottom of transwell plate. Data represent mean ± SEM of n = 3 biologically independent samples.
Extended Data Fig. 8 Characterization of host cells infiltrating MASTER.
A) Timeline of experiment B) Representative FACS plot showing efficient engraftment of human PBMCs (Hu-CD45 + CD3+) in blood. C-D) Different subsets of mouse and human cells that infiltrated MASTER. Cells were gated on live cells. Data in d-e represent mean ± SEM and median of three biologically independent samples. E) Phenotype of the engrafted human T cells that infiltrated into the scaffold. Cells were gated on human-CD45 + CD3 + cells. Data represent mean ± SEM of four biologically independent samples F) FACS plot showing no GFP expression in cells infiltrating MASTER, in blood and in the skin surrounding the scaffold.
Extended Data Fig. 9 Similar numbers of exhausted cells in blood of mice with conventional and MASTER-generated CAR-T cells.
Immunophenotypic composition of CAR-T cells in blood of mice treated with conventionally expanded CAR-T cells i.v. (A) or MASTER (B) at day 12 and 22 post tumor inoculation. Data in a represent mean ± SD of six experimental replicates. Data in b represent mean ± SEM of five experimental replicates.
Extended Data Fig. 10 Subcutaneously implanted MASTER exhibited better control of tumor growth and extended survival compared under stressed dose conditions.
A) Timeline of the study B) In vivo tumor bioluminescence imaging (BLI) of NSG mice (n = 6) treated with MASTER, conventional CAR-T cells, or control non-transduced (NT) cells. Mice were treated with 0.5 ×106 (left), 0.25 ×106 (middle), or 0.125 ×106 (right) CAR T cells. PBMCs seeded onto MASTER were normalized to transduction efficiency and both groups (MASTER and CAR T, i.v) were treated with equivalent number of CAR T cells. C-E) Survival of mice shown as Kaplan- Meier curves. **p < 0.01; ***p < 0.001 Log-rank (Mantel-Cox) test, Gehan-Breslow-Wilcoxon test.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9.
Supplementary Data 1
Source Data for Supplementary Figures.
Source data
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Full-resolution confocal image ×10.
Source Data Extended Data Fig. 2
Full-resolution confocal image ×40.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 9
Statistical Source Data.
Source Data Extended Data Fig. 10
Statistical Source Data.
Rights and permissions
About this article
Cite this article
Agarwalla, P., Ogunnaike, E.A., Ahn, S. et al. Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells. Nat Biotechnol 40, 1250–1258 (2022). https://doi.org/10.1038/s41587-022-01245-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-022-01245-x
This article is cited by
-
Acellular scaffold-based approach for in situ genetic engineering of host T-cells in solid tumor immunotherapy
Military Medical Research (2024)
-
The therapeutic potential of immunoengineering for systemic autoimmunity
Nature Reviews Rheumatology (2024)
-
Biomaterials to enhance adoptive cell therapy
Nature Reviews Bioengineering (2024)
-
In vivo manufacture and manipulation of CAR-T cells for better druggability
Cancer and Metastasis Reviews (2024)
-
Challenges and new technologies in adoptive cell therapy
Journal of Hematology & Oncology (2023)