Abstract
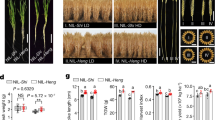
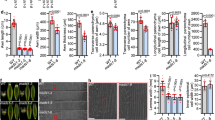
New cultivars with very erect leaves, which increase light capture for photosynthesis and nitrogen storage for grain filling, may have increased grain yields1. Here we show that the erect leaf phenotype of a rice brassinosteroid–deficient mutant, osdwarf4-1, is associated with enhanced grain yields under conditions of dense planting, even without extra fertilizer. Molecular and biochemical studies reveal that two different cytochrome P450s, CYP90B2/OsDWARF4 and CYP724B1/D11, function redundantly in C-22 hydroxylation, the rate-limiting step of brassinosteroid biosynthesis. Therefore, despite the central role of brassinosteroids in plant growth and development, mutation of OsDWARF4 alone causes only limited defects in brassinosteroid biosynthesis and plant morphology. These results suggest that regulated genetic modulation of brassinosteroid biosynthesis can improve crops without the negative environmental effects of fertilizers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sinclair, T.R. & Sheehy, J.E. Erect leaves and photosynthesis in rice. Science 283, 1455 (1999).
Evans, L.T. Crop Evolution, Adaptation and Yield (Cambridge Univ. Press, Cambridge, 1993).
Peng, J. et al. 'Green revolution' genes encode mutant gibberellin response modulators. Nature 400, 256–261 (1999).
Sasaki, A. et al. Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416, 701–702 (2002).
Sakamoto, T. et al. Genetic manipulation of gibberellin metabolism in transgenic rice. Nat. Biotechnol. 21, 909–913 (2003).
Sakamoto, T. & Matsuoka, M. Generating high-yielding varieties by genetic manipulation of plant architecture. Curr. Opin. Biotech. 15, 144–147 (2004).
Li, J. & Chory, J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90, 929–938 (1997).
Yamamuro, C. et al. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12, 1591–1606 (2000).
Hong, Z. et al. Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J. 32, 495–508 (2002).
Hong, Z. et al. A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Plant Cell 15, 2900–2910 (2003).
Choe, S. et al. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell 10, 231–243 (1998).
Bancos, S. et al. Regulation of transcript levels of the Arabidopsis cytochrome P450 genes involved in brassinosteroid biosynthesis. Plant Physiol. 130, 504–513 (2002).
Tanabe, S. et al. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17, 776–790 (2005).
Fujioka, S., Takatsuto, S. & Yoshida, S. An early C-22 oxidation branch in brassinosteroid biosynthetic pathway. Plant Physiol. 130, 930–939 (2002).
Mann, C.C. Crop scientists seek a new revolution. Science 283, 310–314 (1999).
Horton, P. Prospects for crop improvement through the genetic manipulation of photosynthesis: morphological and biochemical aspects of light capture. J. Exp. Bot. 51, 475–485 (2000).
Bishop, G.J. et al. The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 96, 1761–1766 (1999).
Choe, S. et al. Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis. Plant J. 26, 573–582 (2001).
Maeda, E. Rate of lamina inclination in excised rice leaves. Physiol. Plant. 18, 813–827 (1965).
Wada, K. et al. Brassinolide and homobrassinolide promotion of lamina inclination of rice seedlings. Plant Cell Physiol. 22, 323–325 (1981).
Neff, M.M. et al. BAS1: A gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc. Natl. Acad. Sci. USA 96, 15316–15323 (1999).
Sakamoto, T. et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 134, 1642–1653 (2004).
Saito, S. et al. Arabidopsis CYP707As encode (+)-abscisic acid 8′-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol. 134, 1439–1449 (2004).
Fujita, S. et al. Arabidopsis CYP90B1 catalyzes the early C-22 hydroxylation of C27, C28, and C29 sterols. Plant J. 45, in press.
Hiei, Y., Ohta, S., Komari, T. & Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of boundaries of the T-DNA. Plant J. 6, 271–282 (1994).
Acknowledgements
T.S. was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Rice Genome Project IP-1010) and a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT). M.U.-T. and H.K. were supported by a grant from the Program for Promotion of Basic Research Activities for Innovation of Biosciences, and M. Matsuoka was supported by a Grant-in-Aid for Centers of Excellence from MEXT. We thank M. Sekimoto, H. Hayashi, N. Kato, K. Izumi and K. Yatsuda for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Metabolism of recombinanat CYP724B1/D11 protein. (PDF 47 kb)
Supplementary Table 2
Morphological characterization of wild-type and osdwarf4-1 mutant plants. (PDF 74 kb)
Supplementary Table 3
Accession numbers used in this paper. (PDF 67 kb)
Rights and permissions
About this article
Cite this article
Sakamoto, T., Morinaka, Y., Ohnishi, T. et al. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat Biotechnol 24, 105–109 (2006). https://doi.org/10.1038/nbt1173
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1173
This article is cited by
-
Identification of genetic loci for flag leaf traits in wheat (Triticum aestivum L.)
Euphytica (2024)
-
EMS-induced mutagenesis in Choy sum (Brassica chinensis var. parachinensis) and selection for low light tolerance using abiotic stress indices
BMC Plant Biology (2023)
-
Coordinated regulation of vegetative phase change by brassinosteroids and the age pathway in Arabidopsis
Nature Communications (2023)
-
Exploratory biological coordinate analysis on 2,4-epi-brassinolide-induced the size change of leaf angle in tobacco seedlings
Plant Growth Regulation (2023)
-
Mapping QTLs Controlling Grain and Leaf Traits in Iranian Wheat Recombinant Inbred Lines
Plant Molecular Biology Reporter (2023)