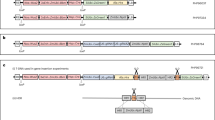
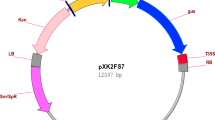
Abstract
Genetic engineering can be used to complement traditional breeding methods in crop plant improvement. Transfer of genes from heterologous species provides the means of selectively introducing new traits into crop plants and expanding the gene pool beyond what has been available to traditional breeding systems. The prerequisites for genetic engineering are efficient transformation and tissue culture systems that allow selection and regeneration of transgenic plants. Cassava, an integral plant for food security in developing countries, has until now been recalcitrant to transformation approaches. We report here a method for regenerating stably transformed cassava plants after cocultivation with Agrobacterium tumefaciens, which opens cassava for future improvement via biotechnology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cock, J.H. 1985. p. 192 in Cassava: new potential fora neglegted crop. Westview Press, Boulder, Colorado.
De Brujin, G.H. and Fresco, L.O. 1989. The importance of cassava in world food production. Neth. J. Agric. Sci. 37: 21–34.
Roca, W.M., Henry, G., Angel, F., and Sarria, R. 1992. Biotechnology research applied to cassava improvement at the International Center of Tropical Agriculture (CIAT). AgBiotech News and Information 4: 303N–308N.
Centro Internacional de Agricultura Tropical. 1980. Cassava. Program annual report 1979. Cali, Colombia.
Food and Agriculture Organization of the United Nations. 1995. FAO Production Yearbook 1994. FAO statistics series 125. 48: 93–94.
Fauquet, C. and Fargtte, D. 1990. African cassava mosaic virus: etiology, epide-mology and control. Plant Dis. 74: 404–411.
Otim-Nape, G.W. 1995. The African cassava mosaic virus (ACMV): a threat to food security in Africa. Proceedings of the Second International Scientific Meeting of The Cassava Biotechnology Network. CIAT working document 150, 2: 519–527.
Rosling, H. 1988. p. 40 in Cassava toxicity and food security. Unicef, African Household Security Programme. Tryck Kontakt, Uppsala, Sweden.
Stamp, J.A. and Henshaw, G.G. 1987. Somatic embryogenesis from clonal leaf tissues of cassava. Ann. Bot. 59: 445–450.
Stamp, J.A. and Henshaw, G.G. 1987. Secondary somatic embryogenesis and plant regeneration in cassava. Plant Cell, Tiss. Org. Cult. 10: 227–233.
Szabados, L., Hoyos, R., and Roca, W. 1987. In vitro somatic embryogenesis and plant regeneration of cassava. Plant Cell Rep. 6: 248–251.
Roca, W.M. and Thro, A.M. (eds.). 1993. Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network. CIAT, Cali, Colombia, working document 123, p. 496.
Cassava Biotechnology Network. 1995. Proceedings of the Second International Scientific Meeting. CIAT working document 150. CIAT, Cali, Colombia. 1: 381.
Schöpke, C., Taylor, N., Carcamo, R., Henshaw, G.G., Beachy, R.N.I., and Fauquet, C.M. 1995. Transformation of cassava embryoids by bombardment of embryogenic suspensions. Proceedings of the Second International Scientific Meeting of The Cassava Biotechnology Network. CIAT working document 150, 1: 257–263.
Sarria, R.A. et al. 1993. Towards the development of Agrobacterium tumefaciens and particle bombardment-mediated cassava transformation, pp. 128–133 in Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network. CIAT Working document, p. 123. Roca, W.M. and Thro, A.M. (eds.).
Schöpke, C., Chavarriaga, P., Fauquet, C.M., and Beachy, R.N. 1993. Cassava tissue culture and transformation: improvement of culture media and the effect of different antibiotics on leaf tissues, pp. 140–145 in Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network. CIAT working document 123. Roca, W.M. and Thro, A.M. (eds.).
Schöpke, C., Franche, C., Bogusz, D., Chavarriaga, P., Fauquet, C.M., and Beachy, R.N. 1993. Transformation in cassava (Manihot esculenta Crantz), pp. 273–289 in Biotechnology in Agriculture and Forestry. Bajaj, Y.P.S. (ed.). Springer-Verlag, Berlin.
Li, H.-Q., Huang, Y.-W., Liang, C.-Y., and Guo, J.-Y. 1995. Improvement of plant regeneration from somatic embryos in cassava. Proceedings of the Second International Scientific Meeting of The Cassava Biotechnology Network. CIAT working document 150. 1: 289–302.
Stamp, J.A. 1987. Somatic embryogenesis in cassava: the anatomy and morphology of the regeneration process. Ann. Bot. 59: 451–459.
Raemakers, C.J.J.M., Jacobsen, E. and Visser, R.G.F. 1995. Histology of somatic embryogenesis and evaluation of somaclonal variation. Proceedings of the Second International Scientific Meeting of The Cassava Biotechnology Network. CIAT working document 150, 1: 336–354.
Calderón, A. 1988. Transformation of Manihot esculents (cassava) using Agrobacterium tumefaciens and expression of the introduced foreign genes in transformed cell lines. MSc thesis, Vrije Universiteit Brussels, Belgium.
Sarria, R., Torres, E., Balcazar, N., Destefano-Beltran, L., and Roca, W.M. 1995. Progress in Agrobacterium-mediateti transformation of cassava (Manihot esculenta Crantz). Proceedings of the Second International Scientific Meeting of The Cassava Biotechnology Network. CIAT working document 150, 1: 241–244.
Chavarriaga-Aguirre, P., Schöpke, C., Sangare, A., Fauquet, C.M., and Beachy, R.N. 1993. Transformation of cassava (Manihot esculenta Crantz) embryogenic tissues using Agrobacterium tumefaciens, pp. 222–228 in Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network. CIAT working document 123. Roca, W.M. and Thro, A.M. (eds.).
Hoekema, A., Hoykaas, P., and Schilperoort, R. 1984. Transfer of octopine T-DNA segment to plant cells mediated by different types of Agrobacterium tumor or root inducing plasmids: generality of virulence systems. J. Bact. 158: 383–385.
Hiei, Y., Ohta, S., Komari, T., and Kumashiro, T. 1994. Efficient transformation of rice (Oryza sativa I.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 6: 271–282.
Holtorf, S., Apel, K., and Bohlmann, H. 1995. Comparison of different constitutive and inducible promoters for the overexpression of transgenes in Arabidopsis thaliana . Plant Mol. Biol. 29: 637–646.
Holsters, M. et al. 1980. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid 3: 212–230.
Sciaky, D., Montoya, A.L., and Chilton, M.D. 1978. Fingerprints of Agrobacterium Ti-plasmids. Plasmid 1: 238–253.
Liu, C.N., Li, X.O., and Gelvin, S.B. 1992. Multiple copies of virG enhance the transient transformation of celery, carrot and rice tissues by Agrobacterium tumefaciens . Plant. Mol. Biol. 20: 1071–1087.
Arias-Garzón, D.I. and Sarria, R. 1995. New Agrobacterium tumefaciens plasmids for cassava transformation. Proceedings of the Second International Scientific Meeting of The Cassava Biotechnology Network. CIAT working document 150, 1: 245–251.
Hood, E.E., Helmer, G.L., Fraley, R.T., and Chilton, M.-D. 1986. The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J. Bacteriol. 168: 1291–1301.
Hood, E.E., Fraley, R.T., and Chilton, M.-D. 1987. Virulence of Agrobacterium tumefaciens A281 on legumes. Plant Physiol. 83: 529–534.
Puonti-Kaerlas, J. 1991. Tissue culture and genetic transformation of pea (Pisum sativum L.). Acta Universitatis Upsaliensis, Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science. Almqvist & Wiksell International, Stockholm.
Zambryski, P. 1988. Basic processes underlying Agrobacterium-mediated DNA transfer to plant cells. Ann. Rev. Genet. 22: 1–30.
Grevelding, C., Fantes, V., Kemper, E., Schell, J., and Masterson, R. 1993. Single-copy T-DNA insertions in Arabidopsis are the predominant form of integration in root-derived transgenics, whereas multiple insertions are found in leaf discs. Plant Mol. Biol. 23: 847–860.
Vancanneyt, G., Schmidt, R., O'Connor-Sanchez, A., Willmitzer, L., and M, R.-S. 1990. Construction of an intron-containing marker, gene splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol. Gen. Genet. 220: 245–250.
Henry, G., Thro, A.-M., and Lynam, J. 1995. Cassava biotechnology priority setting: old hat for a new tool. Proceedings of the Second International Scientific Meeting of The Cassava Biotechnology Network. ClATworking document 150. 1: 46.1–46.18
Vaeck, M. et al. 1987. Transgenic plants protected from insect attack. Nature 327: 239–247.
Fischhoff, D.A. et al. 1987. Insect tolerant transgenic tomato plants. Bio/Technology 5: 807–813.
Perlak, F.J. et al. 1990. Insect resistant cotton plants. Bio/Technology 8: 939–943.
Wünn, J. et al. 1996. Transgenic indica rice breeding line IR58 expressing a synthetic crylA(b) gene from Bacillus thurigiensis provides affective insect pest control. Bio/Technology 14: 171–176.
Fauquet, C.M., Schöpke, C., Chavarriaga-Aguirre, P., Sangare, A., and Beachy, R.N. 1993. Genetic engineering technologies to control viruses and their application to cassava viruses, pp. 190–207 in Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network. CIAT working document 123. Roca, W.M. and Thro, A.M. (eds.).
Beachy, R.N., Loesch-Fries, S., and Turner, N.E. 1990. Coat protein-mediated resistance against virus infection. Annu. Rev. Phytopathol. 28: 451–474.
Wilson, T.M.A. 1993. Strategies to protect crop plants against viruses: pathogen-derived resistance blossoms. Proc. Natl. Acad. Sci. USA 90: 3134–3141.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant. 15: 473–497.
Bevan, M.W., Flavell, R.B. and Chilton, M.-D. 1983. A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 304: 184–187.
Chilton, M.-D. et al. 1974. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc. Natl. Acad. Sci USA 71: 3672–3676.
Mendel, R.R., Müller, B., Schulze, J., Kolesnikov, V., and Zelenin, A. 1989. Delivery of foreign genes to intact barley cells by high velocity microprojectiles. Theor. Appl. Gen. 78: 31–34.
Puonti-Kaerlas, J., Eriksson, T., and Engström, P. 1992. Inheritance of a bacterial hygromycin phosphotransferase gene in the progeny of primary transgenic pea plants. Theor. Appl. Gen. 84: 443–450.
Maniatis, T., Fritsch, E.F., and Sambrook, J. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
Feinberg, A.P. and Vogelstein, B. 1983. A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 132: 6–13.
Fütterer, J. et al. 1995. Standard molecular techniques for the analysis of transgenic plant, pp. 213–263 in Gene transfer to plants. Potrykus, I. and Spangenberg, G. (eds.). Springer-Verlag, Berlin.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, HQ., Sautter, C., Potrykus, I. et al. Genetic transformation of cassava (Manihot esculenta Crantz). Nat Biotechnol 14, 736–740 (1996). https://doi.org/10.1038/nbt0696-736
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0696-736
This article is cited by
-
A cassava common mosaic virus vector for virus-induced gene silencing in cassava
Plant Methods (2021)
-
A system for rapid gene introgression into cassava immature leaves and subsequent recovery of transformed lines
Plant Biotechnology Reports (2021)
-
The potential of using biotechnology to improve cassava: a review
In Vitro Cellular & Developmental Biology - Plant (2016)
-
Somatic embryogenesis and plant regeneration of cassava (Manihot esculenta Crantz) landraces from Cameroon
SpringerPlus (2015)
-
An efficient protocol for Agrobacterium-mediated transformation of the biofuel plant Jatropha curcas by optimizing kanamycin concentration and duration of delayed selection
Plant Biotechnology Reports (2015)