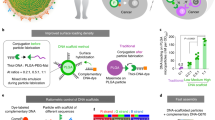
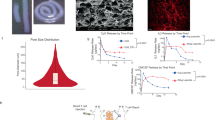
Abstract
Implanting materials in the body to program host immune cells is a promising alternative to transplantation of cells manipulated ex vivo to direct an immune response, but doing so requires a surgical procedure. Here we demonstrate that high-aspect-ratio, mesoporous silica rods (MSRs) injected with a needle spontaneously assemble in vivo to form macroporous structures that provide a 3D cellular microenvironment for host immune cells. In mice, substantial numbers of dendritic cells are recruited to the pores between the scaffold rods. The recruitment of dendritic cells and their subsequent homing to lymph nodes can be modulated by sustained release of inflammatory signals and adjuvants from the scaffold. Moreover, injection of an MSR-based vaccine formulation enhances systemic helper T cells TH1 and TH2 serum antibody and cytotoxic T-cell levels compared to bolus controls. These findings suggest that injectable MSRs may serve as a multifunctional vaccine platform to modulate host immune cell function and provoke adaptive immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jähnisch, H. et al. Dendritic cell-based immunotherapy for prostate cancer. Clin. Dev. Immunol. 2010, 517493 (2010).
Porter, D.L., Levine, B.L., Kalos, M., Bagg, A. & June, C.H. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Ali, O.A., Huebsch, N., Cao, L., Dranoff, G. & Mooney, D.J. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 8, 151–158 (2009).
Ali, O.A., Emerich, D., Dranoff, G. & Mooney, D.J. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci. Transl. Med. 1, ra19 (2009).
Stephan, M.T., Moon, J.J., Um, S.H., Bershteyn, A. & Irvine, D.J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 16, 1035–1041 (2010).
Moon, J.J. et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 10, 243–251 (2011).
St. John, A.L., Chan, C.Y., Staats, H.F., Leong, K.W. & Abraham, S.N. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat. Mater. 11, 250–257 (2012).
Park, J. et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 11, 895–905 (2012).
Engelmayr, G.C. et al. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 7, 1003–1010 (2008).
Freed, L.E. et al. Biodegradable polymer scaffolds for tissue engineering. Biotechnol. (N.Y.) 12, 689–693 (1994).
Place, E.S., George, J.H., Williams, C.K. & Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 38, 1139–1151 (2009).
Lutolf, M. & Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 23, 47–55 (2005).
Yamada, Y. et al. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 10, 955–964 (2004).
Yuen, W.W., Du, N.R., Chan, C.H., Silva, E.A. & Mooney, D.J. Mimicking nature by codelivery of stimulant and inhibitor to create temporally stable and spatially restricted angiogenic zones. Proc. Natl. Acad. Sci. USA 107, 17933–17938 (2010).
Xia, T. et al. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano 3, 3273–3286 (2009).
Kim, J. et al. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew. Chem. Int. Ed. Engl. 47, 8438–8441 (2008).
Li, Z., Barnes, J.C., Bosoy, A., Stoddart, J.F. & Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 41, 2590–2605 (2012).
Garcia-Bennett, A.E. Synthesis, toxicology and potential of ordered mesoporous materials in nanomedicine. Nanomedicine 6, 867–877 (2011).
Petushkov, A., Ndiege, N., Salem, A.K. & Larsen, S.C. Toxicity of silica nanomaterials: Zeolites, mesoporous silica, and amorphous silica nanoparticles. Adv. Mol. Toxicol. 4, 223–266 (2010).
Zhao, D., Huo, Q., Feng, J., Chmelka, B.F. & Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 120, 6024–6036 (1998).
Schmidt-Winkel, P., Yang, P., Margolese, D.I., Chmelka, B.F. & Stucky, G.D. Fluoride-induced hierarchical ordering of mesoporous silica in aqueous acid-syntheses. Adv. Mater. 11, 303–307 (1999).
Hudson, S.P., Padera, R.F., Langer, R. & Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 29, 4045–4055 (2008).
He, Q., Shi, J., Zhu, M., Chen, Y. & Chen, F. The three-stage in vitro degradation behavior of mesoporous silica in simulated body fluid. Microporous Mesoporous Mater. 131, 314–320 (2010).
Cauda, V., Schlossbauer, A. & Bein, T. Bio-degradation study of colloidal mesoporous silica nanoparticles: effect of surface functionalization with organo-silanes and poly (ethylene glycol). Microporous Mesoporous Mater. 132, 60–71 (2010).
Dieu, M. et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188, 373–386 (1998).
Randolph, G.J., Ochando, J. & Partida-Sánchez, S. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 26, 293–316 (2008).
Steinman, R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9, 271–296 (1991).
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Medzhitov, R. & Janeway, C.A. Jr. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9, 4–9 (1997).
Klinman, D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 4, 249–259 (2004).
Victora, G.D. & Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 30, 429–457 (2012).
Crotty, S. Follicular helper CD4 T cells (Tfh). Annu. Rev. Immunol. 29, 621–663 (2011).
Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 21, 1171–1178 (2003).
Russell, B., Desai, T.A. & Goldspink, P. Temporal release of growth factors from 3d micro rod scaffolds for tissue regeneration. EP 2170419 A1 (2010).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Sharp, F.A. et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. USA 106, 870–875 (2009).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Zhang, H. et al. Processing pathway dependence of amorphous silica nanoparticle toxicity: colloidal vs pyrolytic. J. Am. Chem. Soc. 134, 15790–15804 (2012).
Reddy, S.T. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 25, 1159–1164 (2007).
Meng, H. et al. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J. Am. Chem. Soc. 132, 12690–12697 (2010).
Yang, P., Shili, G. & Jun, L. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 41, 3679–3698 (2012).
Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 5, 263–274 (2005).
Rabinovich, G.A., Gabrilovich, D. & Sotomayor, E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25, 267–296 (2007).
Moon, J.J. et al. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc. Natl. Acad. Sci. USA 109, 1080–1085 (2012).
Zhang, X.Q. et al. Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles. J. Immunother. 30, 469–478 (2007).
Uto, T. et al. The induction of innate and adaptive immunity by biodegradable poly (γ-glutamic acid) nanoparticles via a TLR4 and MyD88 signaling pathway. Biomaterials 32, 5206–5212 (2011).
Hutchison, S. et al. Antigen depot is not required for alum adjuvanticity. FASEB J. 26, 1272–1279 (2012).
Lu, A., Schmidt, W., Spliethoff, B. & Schüth, F. Synthesis and characterization of nanocast silica NCS-1 with CMK-3 as a template. Chemistry 10, 6085–6092 (2004).
Faulkner, L., Buchan, G. & Baird, M. Interleukin-10 does not affect phagocytosis of particulate antigen by bonemarrow-derived dendritic cells but does impair antigen presentation. Immunology 99, 523–531 (2000).
Bhattacharya, P., Gopisetty, A., Ganesh, B.B., Sheng, J.R. & Prabhakar, B.S. GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J. Leukoc. Biol. 89, 235–249 (2011).
Acknowledgements
This work was supported by the National Institutes of Health (NIH) (1R01EB015498), the National Science Foundation (NSF) Graduate Research Fellowship Program (GRFP), the Wyss Institute for Biologically Inspired Engineering at Harvard University, and National Research Foundation (NRF) grants (2012R1A1A1042735, 2010-0027955) funded by the National Research Foundation under the Ministry of Science, ICT & Future Planning, Korea. We also thank Dana-Farber/Harvard Cancer Center (DF/HCC) Research Pathology Core and R. Bronson for the examination of the histology slides and J. Weaver from the Wyss Institute of Biologically Inspired Engineering for his help with scanning electron microscopy (SEM). Lastly, we thank R. Betensky from the Department of Biostatistics, Harvard School of Public Health and the Harvard Catalyst for her help with statistical analysis; Harvard Catalyst is supported, in part, by the NIH (UL1 TR001102).
Author information
Authors and Affiliations
Contributions
J.K., W.A.L. and D.J.M. conceived and designed the experiments. J.K., W.A.L., Y.C., S.A.L. and C.S.V. performed the experiments. J.K., W.A.L., G.D. and D.J.M. analyzed the data. J.K., W.A.L. and D.J.M. wrote the manuscript. All authors discussed the results and commented on the manuscript. J.K. and W.A.L. contributed equally to this work. The principal investigator is D.J.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 (PDF 1774 kb)
Supplementary Video 1
Z-stack of confocal images of sectioned nodule retrieved at day 7 post-injection. The injected MSRs were labeled with AF488 and load with GM-CSF (1 μg). The cross-section was stained with DAPI and phalloidin. (MOV 7043 kb)
Supplementary Video 2
Z-stack of confocal images of sectioned nodule retrieved at day 28 post-injection. The injected MSRs were labeled with AF488 and load with GM-CSF (1 μg). The cross-section was stained with DAPI and phalloidin. (MOV 2628 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Li, W., Choi, Y. et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol 33, 64–72 (2015). https://doi.org/10.1038/nbt.3071
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3071
This article is cited by
-
Immunization against Zika by entrapping live virus in a subcutaneous self-adjuvanting hydrogel
Nature Biomedical Engineering (2023)
-
Targeted modulation of immune cells and tissues using engineered biomaterials
Nature Reviews Bioengineering (2023)
-
Adoptive T cell transfer and host antigen-presenting cell recruitment with cryogel scaffolds promotes long-term protection against solid tumors
Nature Communications (2023)
-
Pattern recognition receptors and their nano-adjuvants for cancer immunotherapy
Journal of Pharmaceutical Investigation (2023)
-
Recent advances in porous nanomaterials-based drug delivery systems for cancer immunotherapy
Journal of Nanobiotechnology (2022)